
ARZOLAN 20 MG/ML COLIRIO EN SOLUCION

Cómo usar ARZOLAN 20 MG/ML COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Arzolan 20 mg/ml colirio en solución
Dorzolamida
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.
Contenido del prospecto
- Qué es Arzolan y para qué se utiliza
- Qué necesita saber antes de empezar a usar Arzolan
- Cómo usar Arzolan
- Posibles efectos adversos
- Conservación de Arzolan
- Contenido del envase e información adicional
1. Qué es Arzolan y para qué se utiliza
Arzolan es un colirio en solución estéril. Arzolan contiene como principio activo dorzolamida, un compuesto relacionado con las sulfamidas.
Dorzolamida es un inhibidor de la anhidrasa carbónica de uso oftálmico y reduce la presión elevada del interior del ojo.
Está indicado en el tratamiento de la presión intraocular elevada en pacientes con hipertensión ocular y glaucoma (glaucoma de ángulo abierto, glaucoma pseudoexfoliativo). Arzolan puede emplearse solo o en combinación con otros medicamentos que disminuyan la presión en el interior del ojo (llamados betabloqueantes).
2. Arzolan
No use Arzolan
- Si es alérgico (hipersensible) a dorzolamida o a cualquiera de los demás componentes de esta solución.
- Si padece problemas graves de riñón.
- Si padece un trastorno de pH (equilibrio ácido/alcalino) en la sangre.
Advertencias y precauciones
Antes de iniciar el tratamiento con Arzolan consulte a su médico:
- Si padece o ha padecido problemas de hígado con anterioridad
- Si se le ha informado que padece un defecto de córnea
- Si ha tenido alguna alergia a cualquier medicamento
- Si se ha sometido o va a someterse a una intervención quirúrgica ocular
- Si ha sufrido una lesión en el ojo o padece una infección ocular
- Si tiene antecedentes de piedras en el riñón
- Si está tomando otro inhibidor de la anhidrasa carbónica
- Si utiliza lentes de contacto (ver la sección “Información importante sobre los componentes de Arzolan”).
- Si presenta cualquier irritación ocular o trastorno ocular nuevo, como enrojecimiento del ojo o hinchazón de los párpados, consulte a su medico de inmediato.
- Si sospecha que Arzolan le está produciendo una reacción alérgica (por ejemplo, erupción cutánea o picor, inflamación ocular) interrumpa su administración y consulte a su médico lo antes posible.
Niños y adolescentes
Arzolan únicamente debe utilizarse en niños si los beneficios justifican los riesgos. Su médico podrá aconsejarle.
Uso de Arzolan con otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta.
En particular debe consultar a su médico si está utilizando otro inhibidor de la anhidrasa carbónica como acetazolamida. Puede que esté utilizando este tipo de medicamento por vía oral, como colirio, o de cualquier otro modo.
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento. Informe a su médico si está embarazada o tiene intención de estarlo. No debe utilizar Arzolan durante el embarazo a menos que su médico así lo recomiende.
No debe utilizar Arzolan durante la lactancia.
Conducción y uso de máquinas
Arzolan puede causar mareo y trastornos visuales en algunos pacientes. No conduzca ni maneje maquinaria hasta que los síntomas hayan desaparecido.
Arzolan contiene cloruro de benzalconio
Este medicamento puede producir irritación ocular porque contiene cloruro de benzalconio.
Evitar el contacto con las lentes de contacto blandas.
Retirar las lentes de contacto antes de la aplicación y esperar al menos 15 minutos antes de volver a colocarlas.
Altera el color de las lentes de contacto blandas.
3. Cómo Arzolan
Siga exactamente las instrucciones de administración de Arzolan indicadas por su médico. Consulte a su médico o farmacéutico si tiene dudas.
La posología apropiada y la duración del tratamiento serán establecidas por su médico.
Cuando Arzolan se utiliza solo, la dosis habitual es de una gota en el ojo u ojos afectados tres veces al día, a saber, por la mañana, por la tarde y por la noche.
Si su médico le ha recomendado el uso de Arzolan con un colirio betabloqueante (medicamentos que disminuyen la presión del interior del ojo), la dosis habitual es de una gota en el ojo u ojos dos veces al día, por ejemplo por la mañana y por la tarde.
Si va a usar Arzolan con otro colirio, las aplicaciones de los distintos medicamentos deben espaciarse al menos 10 minutos. De igual modo, cuando vaya a sustituir otro medicamento de colirio por Arzolan, utilizado para disminuir la presión en el ojo, debe interrumpir la administración del otro medicamento tras tomar la dosis apropiada de un día e iniciar la administración de Arzolan, al día siguiente.
No cambie la dosis del medicamento sin consultar al médico. Si debe interrumpir el tratamiento consulte a su médico de inmediato.
No deje que la punta del recipiente toque los ojos o las zonas que los rodean. Puede estar contaminada con bacterias capaces de causar infecciones oculares que originen graves daños en los ojos, e incluso la pérdida de la visión. Para evitar una posible contaminación del recipiente, evite que la punta del recipiente entre en contacto con cualquier superficie.
Instrucciones de uso:
Se recomienda lavar las manos antes de aplicar el colirio.
Sitúese, si es posible, delante de un espejo para facilitar la aplicación.
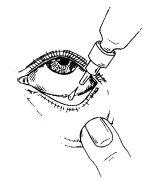
- Antes de utilizar el medicamento por primera vez, asegúrese de que la tira de seguridad en el cuello del frasco esté intacta. Cuando el frasco no se ha abierto aún, es normal la existencia de un espacio entre el frasco y el capuchón.
- Quite el capuchón del frasco.
- Incline la cabeza hacia atrás y separe el párpado inferior ligeramente, formando una pequeña separación entre el párpado y el ojo.
- Invierta el frasco y presione hasta dispensar una sola gota en el ojo de acuerdo con las instrucciones de su médico. NO TOQUE EL OJO NI EL PÁRPADO CON LA PUNTA DEL GOTERO.
- Cierre el ojo y presione con el dedo la esquina interna del ojo con su dedo durante aproximadamente dos minutos. Esto ayuda a evitar que el medicamento llegue al resto del cuerpo.
- Repita los pasos 3 y 4 en el otro ojo si así se lo ha indicado su médico.
- Vuelva a colocar el capuchón y cierre el frasco inmediatamente después de usarlo.
Si usa másArzolandel que debiera
Si se aplican demasiadas gotas en el ojo o traga parte del contenido del frasco, consulte a su médico de inmediato.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20.
Si olvidó usarArzolan
Es importante administrar Arzolan como le ha indicado el médico.
Si olvida aplicar una dosis, debe administrársela lo antes posible. Sin embargo, si es casi la hora de la siguiente dosis, no se administre la dosis olvidada y continúe con el programa de dosis previsto normalmente.
No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Arzolan
Arzolan debe utilizarse a diario para que surta el efecto deseado. Si debe interrumpir el tratamiento, consulte a su médico de inmediato.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Arzolan puede producir efectos adversos, aunque no todas las personas los sufran.
Los siguientes términos se utilizan para describir con qué frecuencia se han comunicado los efectos adversos:
Muy frecuentes | Pueden afectar a más de 1 de cada 10 pacientes |
Frecuentes | Pueden afectar hasta 1 de cada 100 pacientes |
Poco frecuentes | Pueden afectar hasta 1 de cada 1.000 pacientes |
Raros | Pueden afectar hasta 1 de cada 10.000 pacientes |
Frecuencia no conocida | No puede estimarse a partir de los datos disponibles |
Puede experimentar alguno o todos los efectos adversos siguientes con Arzolan
Trastornos oculares:
Muy frecuentes: quemazón y escozor
Frecuentes: inflamación o hinchazón de la superficie del ojo u ojos y posible inflamación del párpado o párpados y/o alrededor del ojo u ojos, lagrimeo o picor ocular, visión borrosa, efectos en la superficie del ojo
Poco frecuentes: inflamación de la capa media del globo ocular
Raros: hinchazón de la superficie del ojo u ojos, desprendimiento coroideo con síntomas de alteraciones/trastornos visuales (después de cirugía de filtración), hipotonía ocular, enrojecimiento del ojo u ojos, dolor ocular, costras en el párpado, miopía transitoria (que remite al cesar la terapia)
Trastornos gastrointestinales:
Frecuentes: náusea, sabor amargo
Raros: irritación de garganta, boca seca
Trastornos generales y alteraciones en el lugar de administración:
Frecuentes: astenia/fatiga
Raros: Hipersensibilidad: signos y síntomas de reacciones locales (reacciones palpebrales) y reacciones alérgicas sistémicas que incluyen hinchazón de la cara, labios, lengua y/o garganta que puede causar dificultad en la respiración o al tragar, urticaria y picor, erupción cutánea, falta de respiración y raramente broncoespasmo (contracción del músculo liso bronquial)
Trastornos del sistema nervioso:
Frecuentes: cefalea
Raros: mareo, entumecimiento/hormigueo
Trastornos renales y urinarios:
Raros: formación de cálculos urinarios
Trastornos respiratorios, torácicos y mediastínicos:
Raros: hemorragia nasal
Trastornos de la piel y del tejido subcutáneo:
Raros: inflamación cutánea
Trastornos cardíacos:
Frecuencia no conocida: latidos cardíacos fuertes que pueden ser rápidos o irregulares (palpitaciones), aumento de la frecuencia cardiaca
Trastornos vasculares:
Frecuencia no conocida: aumento de la tensión arterial
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte con su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de uso humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
5. Conservación de Arzolan
Mantener fuera de la vista y del alcance de los niños.
No utilice Arzolan después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar el frasco en el embalaje exterior para protegerlo de la luz.
Conservar por debajo de 30°C.
Arzolan debe utilizarse en un plazo no superior a 28 días después de la apertura inicial del frasco. Por lo tanto, debe desechar el frasco 4 semanas después de haberlo abierto por primera vez, aunque quede solución. Como recordatorio, anote la fecha de apertura de cada frasco en el espacio de la caja.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Arzolan
Composición deArzolan
- El principio activo es dorzolamida. Cada ml contiene 20 mg de dorzolamida (equivalente a dorzolamida hidrocloruro).
- Los demás componentes son manitol, hidroxietil celulosa, cloruro de benzalconio (como conservante), citrato de sodio, hidróxido de sodio para ajustar el pH y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Arzolan es una solución estéril, isotónica, tamponada incolora, ligeramente viscosa en un frasco de polietileno de baja densidad opaco y blanco con un gotero sellado y una tapa compuesta de 2 piezas. Cada frasco contiene 5 ml del colirio en solución.
Arzolan está disponible en envases de 1 frasco con 5 ml de colirio en solución.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Tiedra Farmacéutica, S.L.
C/ Colón, 7
30510 Yecla (Murcia)
España
Responsable de la fabricación:
Pharmathen S.A.
6, Dervenakion str.
153 51 Pallini Attiki,
Grecia
Fecha de la última revisión de este prospecto: Enero 2023
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia5.12 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ARZOLAN 20 MG/ML COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 20 mg/mlPrincipio activo: dorzolamidaFabricante: Brill Pharma S.L.Requiere recetaForma farmacéutica: COLIRIO, 2%Principio activo: dorzolamidaFabricante: Aristo Pharma Iberia S.L.Requiere recetaForma farmacéutica: COLIRIO, 2%Principio activo: dorzolamidaFabricante: Farmalider S.A.Requiere receta
Médicos online para ARZOLAN 20 MG/ML COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ARZOLAN 20 MG/ML COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















