ALZERTA DOS POR SEMANA 4,6 MG/24 H PARCHES TRANSDERMICOS

Cómo usar ALZERTA DOS POR SEMANA 4,6 MG/24 H PARCHES TRANSDERMICOS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Alzerta dos por semana 4,6 mg/24 h parches transdérmicos
Alzerta dos por semana 9,5 mg/24 h parches transdérmicos
Rivastigmina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Alzerta dos por semana y para qué se utiliza
- Qué necesita saber antes de empezar a usar Alzerta dos por semana
- Cómo usar Alzerta dos por semana
- Posibles efectos adversos
- Conservación de Alzerta dos por semana
- Contenido del envase e información adicional
1. Qué es Alzerta dos por semana y para qué se utiliza
El principio activo de Alzerta dos por semana es rivastigmina.
La rivastigmina pertenece al grupo los inhibidores de la colinesterasa. En pacientes con demencia Alzheimer, determinadas células nerviosas mueren en el cerebro, provocando bajos niveles del neurotransmisor acetilcolina (una sustancia que permite que las células nerviosas se comuniquen entre ellas). La rivastigmina actúa bloqueando las enzimas que rompen la acetilcolina: acetilcolinesterasa y butirilcolinesterasa. Bloqueando estas enzimas, rivastigmina permite el aumento de acetilcolina en el cerebro, ayudando a reducir los síntomas de la enfermedad de Alzheimer.
Alzerta dos por semana se utiliza para el tratamiento de pacientes adultos con demencia de Alzheimer de leve a moderadamente grave, un trastorno progresivo del cerebro que afecta gradualmente a la memoria, capacidad intelectual y el comportamiento.
2. Qué necesita saber antes de empezar a usar Alzerta dos por semana
No use Alzerta dos por semana
- si es alérgico a la rivastigmina (el principio activo de Alzerta dos por semana) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si alguna vez ha tenido una reacción alérgica a un medicamento similar (derivados del carbamato).
- si tiene una reacción de la piel que se extiende más allá del tamaño del parche, si hay una reacción local más intensa (tales como ampollas, inflamación de la piel, hinchazón) y si no hay mejoría durante las 48 horas después de retirar el parche transdérmico.
Si se encuentra en algunas de estas situaciones, informe a su médico y no utilice Alzerta dos por semana parches transdérmicos.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Alzerta dos por semana:
- si tiene o ha tenido alguna vez algún problema cardíaco como ritmo cardíaco (pulso) irregular o lento, prolongación de QTc, antecedentes familiares de prolongación de QTc, torsade de pointes, o si tiene un nivel bajo en sangre de potasio o de magnesio.
- si tiene o ha tenido alguna vez úlcera de estómago activa.
- si tiene o ha tenido alguna vez dificultades al orinar.
- si tiene o ha tenido alguna vez convulsiones.
- si tiene o ha tenido alguna vez asma o una enfermedad respiratoria grave.
- si sufre temblores.
- si tiene peso corporal bajo.
- si tiene reacciones gastrointestinales tales como sensación de náuseas, vómitos y diarrea. Podría deshidratarse (pérdida de gran cantidad de fluido) si los vómitos o diarrea son prolongados.
- si tiene problemas del hígado (insuficiencia hepática).
Si se encuentra en alguna de estas situaciones, puede que su médico considere necesario realizar un mayor seguimiento mientras esté en tratamiento.
Si no ha utilizado los parches durante más de tres días, no se ponga otro sin antes consultarlo con su médico.
Retire con cuidado cualquier parche antes de poner uno de nuevo. No aplique más de un parche a la vez. Llevar múltiples (o más de uno) parches transdérmicos en su cuerpo, podría exponerlo a una mayor cantidad de este medicamento de la que debería.
Niños y adolescentes
No existe experiencia del uso de este medicamento en la población pediátrica en el tratamiento de la enfermedad de Alzheimer.
Otros medicamentos y Alzerta dos por semana
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Este medicamento podría interferir con medicamentos anticolinérgicos algunos de los cuales son medicamentos utilizados para aliviar los calambres o espasmos estomacales (p.ej. diciclomina), para el tratamiento de la enfermedad de Parkinson (p.ej. amantadina) o para prevenir los mareos por movimiento (p.ej. difenhidramina, escopolamina, o meclizina).
Este medicamento no se debe administrar al mismo tiempo que metoclopramida (un medicamento utilizado para aliviar o prevenir las náuseas y los vómitos). La toma de los dos medicamentos juntos puede causar problemas como rigidez en las extremidades y temblor de manos.
En caso de que tenga que someterse a una intervención quirúrgica mientras está utilizando este medicamento, informe a su médico de que lo está utilizando, ya que puede potenciar excesivamente los efectos de algunos relajantes musculares de la anestesia.
Se debe tener precaución cuando se utiliza este medicamento junto con beta bloqueantes (medicamentos como atenolol utilizados para tratar la hipertensión, angina y otras afecciones cardíacas). La toma de los dos medicamentos juntos puede causar complicaciones como el descenso de la frecuencia cardíaca (bradicardia) que puede dar lugar a desmayos o pérdidas de conciencia.
Se debe tener precaución cuando se utiliza Alzerta dos por semana junto con otros medicamentos que pueden afectar el ritmo cardíaco o el sistema eléctrico del corazón (prolongación QT).
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Si está embarazada es necesario evaluar los beneficios del uso de este medicamento frente a los posibles efectos adversos para el feto. No se debe utilizar este medicamento durante el embarazo a menos que sea claramente necesario.
No debe dar el pecho durante su tratamiento con este medicamento.
Conducción y uso de máquinas
Su médico le informará si su enfermedad le permite conducir o utilizar maquinaria de manera segura. Este medicamento puede causar mareos y confusión grave. Si se siente mareado o confuso no conduzca ni utilice maquinaria ni desarrolle otras tareas que requieran su atención.
3. Cómo usar Alzerta dos por semana
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cómo iniciar el tratamiento
Su médico le indicará la dosis de este medicamento más adecuada en su caso.
- Normalmente se comienza el tratamiento con Alzerta dos por semana 4,6 mg/24 h.
- La dosis diaria habitual recomendada es 9,5 mg/24 h. Si esta dosis es bien tolerada, el médico que lo trata puede considerar incrementar la dosis a 13,3 mg/24 h. La dosis de 13,3 mg/24 h no puede alcanzarse con Alzerta dos por semana. Para estados en que se deba utilizar esta dosificación, se encuentran disponibles otros parches de rivastigmina que contienen la dosis de 13,3 mg/24h.
- Lleve sólo un parche transdérmico rectangulary una cubierta adhesiva oval al mismo tiempo (como se detalla más abajo) y sustitúyalos dos veces por semana, como máximo después de 4 días.
Debe cambiar los parches en dos días fijos:
Cada uno en
Lunes y Viernes O
Martes y Sábado O
Miércoles y Domingo O
Jueves y Lunes O
Viernes y Martes O
Sábado y Miércoles O
Domingo y Jueves.
Cambie siempre los parches a la misma hora del día. Como recordatorio, debe anotar estos días y la hora del día.
Durante el tratamiento, su médico podría ajustar la dosis dependiendo de sus necesidades individuales.
Si no ha utilizado los parches durante más de tres días, no se ponga otro antes de que lo haya consultado a su médico. El tratamiento con parche transdérmico se puede reiniciar a la misma dosis si el tratamiento no se interrumpe durante más de tres días. De lo contrario, su médico le hará reiniciar su tratamiento con Alzerta dos por semana 4,6 mg/24 h.
Este medicamento se puede utilizar con alimentos, bebida y alcohol.
Dónde colocar su parche Alzerta dos por semana transdérmico
- Antes de ponerse un parche, asegúrese que la piel esté limpia, seca y sin pelo, sin polvos, aceite, hidratante o loción que impidan que el parche se pegue bien a la piel, sin cortes, enrojecimientos o irritaciones.
- Quítese cuidadosamente cualquier parche que lleve antes de ponerse uno nuevo.El llevar múltiples parches en su cuerpo podría exponerlo a una cantidad excesiva de este medicamento y esto podría ser potencialmente peligroso.
- Póngase UNparche transdérmico rectangular junto con una cubierta adhesiva en UNA SOLAde las posibles zonas como se muestra en los siguientes diagramas:
- parte superior del brazo izquierdo oderecho
- parte superior izquierda osuperior derecha del pecho (evitando los pechos)
- parte superior izquierda osuperior derecha de la espalda
- parte inferior izquierda oinferior derecha de la espalda
Después de 4 días como máximo quítese el parche previo antes de ponerse UN parche nuevo en SOLO UNA de las siguientes zonas posibles. |
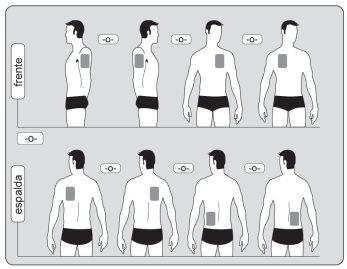
Cada vez que se cambie el parche, debe quitarse el parche anterior antes de ponerse el nuevo parche en un lugar diferente de la piel (por ejemplo en el lado derecho del cuerpo durante cuatro días, y luego en el lado izquierdo durante tres días, o durante cuatro días en la parte de superior del cuerpo y posteriormente en la parte inferior del cuerpo durante tres días). Espere al menos 14 días para volver a ponerse un parche nuevo exactamente en la misma área de piel.
Cómoaplicar su Alzerta dos por semana parche transdérmico
Alzerta dos por semana es para uso transdérmico.
Los parches de Alzerta dos por semana están formados de dos partes:
- un parche rectangular, traslúcido, que contiene la sustancia activa (parche transdérmico) sellado en un sobre y
- un parche oval, beige, sin sustancia activa (cubierta adhesiva) también sellado en un sobre. Este sobre es mayor que el sobre que contiene el parche transdérmico.
| |
Parche transdérmico que contiene la sustancia activa | Parche de tela sin sustancia activa (para la fijación) |
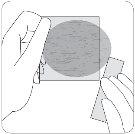
No abra el sobre ni saque el parche hasta el momento de ponérselo.
La aplicación siemprese empieza con el parche transdérmico rectangular.
| Quítese cuidadosamente el parche existente antes de ponerse uno nuevo. Los pacientes que inician el tratamiento por primera vez y para pacientes que reinician el tratamiento con rivastigmina después de la interrupción del tratamiento, deben empezar por la segunda figura. |
| Cada parche transdérmico se encuentra sellado en un sobre protector individual. Sólo se debe abrir el sobre cuando vaya a ponerse el parche. Corte el sobre por ambas marcas de tijeras, pero no más allá de la línea indicada. Abrir el sobre. No corte toda la longitud del sobre para evitar dañar el parche. Saque el parche transdérmico rectangular y traslúcido del sobre. |
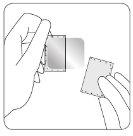
| Una lámina protectora cubre el lado adhesivo del parche. Quite la primera hoja de la lámina protectora sin tocar con los dedos el lado adhesivo del parche. |
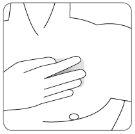
| Coloque el lado adhesivo del parche sobre la parte superior o inferior de la espalda o en la parte superior del brazo o en el pecho y a continuación quite la segunda hoja de la lámina protectora. |
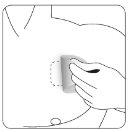
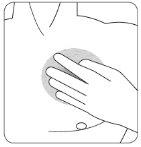
| Presione firmemente el parche contra la piel con la palma de la mano durante unos 15 segundos y asegúrese de que los bordes se han pegado bien. |
Continúe con la aplicación de la cubierta adhesiva ovalada. | |
| Corte el segundo sobre, más grande por ambas marcas de tijeras, pero no más allá de la línea indicada. Abrir el sobre. No corte toda la longitud del sobre para evitar dañar el parche. Saque la cubierta adhesiva oval y beige del sobre. |
| Una lámina protectora cubre el lado adhesivo de la cubierta. Quite la primera hoja del lado más pequeño de la lámina protectora sin tocar con los dedos el lado adhesivo. |
| Coloque la cubierta adhesiva con el lado adhesivo sobre el parche transdérmico, de manera que el parche quede totalmente cubierto y a continuación quite la segunda hoja de la lámina protectora. |
| Presione firmemente el parche contra la piel con la palma de la mano durante un mínimo de 30 segundos y asegúrese de que los bordes se han pegado bien. |
Si esto le ayuda, puede escribir sobre la cubierta adhesiva, por ejemplo, el día de la semana, con un bolígrafo de punta fina redondeada.
Debe llevar puesto el parche continuamente hasta el momento de cambiarlo por otro nuevo. Cuando se ponga un nuevo parche, puede probar con diferentes zonas para encontrar las que le resulten más cómodas y donde la ropa no roce con el parche.
Cómo quitar su Alzerta dos por semana parche transdérmico
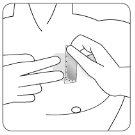
Tire suavemente de uno de los bordes de la cubierta adhesiva del parche para despegarlo lentamente junto con el parche de la piel.
Si el parche transdérmico permanece en la piel, tire suavemente de un borde hasta que se desprenda por completo de la piel.
Si quedan residuos adhesivos sobre la piel, empape el área con agua tibia y jabón suave o utilice aceite de bebé para eliminarlo. No se debe utilizar alcohol u otros líquidos disolventes (quitaesmaltes de uñas u otros disolventes).
Después de retirar el parche, se debe lavar las manos con jabón y agua. En caso de contacto con los ojos o si los ojos se enrojecen después de manipular el parche, se debe lavar inmediatamente con abundante agua y pedir consejo médico si los síntomas no se resuelven.
¿Puede llevar su Alzerta dos por semana parche transdérmico cuando se bañe, nade o se exponga al sol?
- El baño, la natación o la ducha no deberían afectar al parche. Asegúrese de que no se despegue mientras realice estas actividades.
- No exponga al parche a una fuente de calor externa (p.ej. luz solar excesiva, sauna, solarium) durante periodos de tiempo largos.
Qué hacer si se le cae un parche
Si se le cayera un parche, póngase uno nuevo, y cámbielo el día/hora habitual.
¿Cuándo y durante cuánto tiempo debe ponerse su Alzerta dos por semana parche transdérmico?
- Para beneficiarse de su tratamiento debe ponerse un nuevo parche dos veces por semana, a más tardar después de cuatro días, preferiblemente a la misma hora del día.
- Lleve sólo un parche transdérmico rectangular y una cubierta adhesiva oval al mismo tiempo y sustituya el parche por otro nuevo dos veces por semana en dos días fijos.
Si usa más Alzerta dos por semana del que debe
Si accidentalmente se ha puesto más de un parche transdérmico rectangular, quite todos los parches de la piel e informe de ello a su médico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20 (indicando el medicamento y la cantidad administrada). Es posible que necesite atención médica. Algunas personas que han tomado accidentalmente cantidades demasiado altas de rivastigmina han tenido sensación de náuseas, vómitos, diarrea, tensión alta y alucinaciones. Pueden producirse también un enlentecimiento de la frecuencia cardiaca y desmayos.
Si olvidó usar Alzerta dos por semana
Si se da cuenta que ha olvidado ponerse un parche, póngaselo inmediatamente, si el tratamiento no se ha interrumpido por más de tres días.
Reemplace este parche a la hora habitual del día habitual, para volver a su pauta de dosificación.
No se ponga dos parches transdérmicos para compensar el que olvidó. Si no ha aplicado un parche durante más de tres días, no aplique el siguiente antes de haber hablado con su médico.
Si interrumpe el tratamiento con Alzerta dos por semana
Informe a su médico o farmacéutico si deja de utilizar los parches.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Puede tener efectos adversos con más frecuencia al empezar su tratamiento o cuando se le aumente la dosis. Generalmente, los efectos adversos lentamente desaparecerán a medida que su organismo vaya acostumbrándose al medicamento.
Si advierte alguno de los siguientes efectos adversos que pueden ser graves, quítese el parche e informe inmediatamente a su médico.
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Pérdida de apetito
- Sensación de mareo
- Sensación de agitación o adormecimiento
- Incontinencia urinaria (imposibilidad de detener adecuadamente la orina).
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- Problemas con el ritmo de su corazón tales como ritmo cardiaco lento
- Ver cosas que realmente no existen (alucinaciones)
- Úlcera de estómago
- Deshidratación (pérdida de gran cantidad de líquido)
- Hiperactividad (alto nivel de actividad, inquietud)
- Agresividad
Raras(pueden afectar hasta 1 de cada 1.000 personas)
- Caídas
Muy raras(pueden afectar hasta 1 de cada 10.000 personas)
- Rigidez de los brazos y piernas
- Temblor en las manos
No conocida(no puede estimarse a partir de los datos disponibles)
- Reacción alérgica donde se aplicó el parche, tales como ampollas o inflamación de la piel
- Empeoramiento de los signos de enfermedad de Parkinson – tales como temblor, rigidez y dificultad de movimiento.
- Síndrome de Pisa (afección que conlleva una contracción muscular involuntaria y la inclinación anormal del cuerpo y la cabeza hacia un lado)
- Inflamación del páncreas – los signos incluyen dolor de la parte alta del estómago, frecuentemente acompañado de sensación de náuseas o vómitos
- Ritmo cardiaco rápido o irregular
- Tensión arterial alta
- Crisis epilépticas (convulsiones)
- Trastornos hepáticos (coloración amarillenta de la piel, amarillamiento del blanco de los ojos, oscurecimiento anormal de la orina o náuseas inexplicables, vómitos, cansancio y pérdida de apetito)
- Cambios en los análisis que muestran el funcionamiento de su hígado
- Sensación de inquietud
- Pesadillas
Si nota alguno de los efectos adversos listados arriba, quítese el parche e informe inmediatamente a su médico.
Otros efectos adversos experimentados con rivastigmina cápsulas o solución oral y que pueden tener lugar con los parches:
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Excesiva saliva
- Pérdida de apetito
- Sensación de agitación
- Sensación de malestar general
- Temblor o sensación de confusión
- Aumento de la sudoración
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- Ritmo cardiaco irregular (p.ej. ritmo cardiaco rápido)
- Dificultad para dormir
- Caídas accidentales
Raras(pueden afectar hasta 1 de cada 1.000 personas)
- Crisis epilépticas (convulsiones)
- Úlcera en el intestino
- Dolor en el pecho – causado probablemente por espasmo en el corazón
Muy raras(pueden afectar hasta 1 de cada 10.000 personas)
- Tensión arterial alta
- Inflamación del páncreas – los signos incluyen dolor grave de la parte alta del estómago frecuentemente con sensación de náuseas o vómitos
- Sangrado gastrointestinal – se manifiesta como sangre en las heces o al vomitar
- Ver cosas que no existen (alucinaciones)
- Algunas personas que han sufrido vómitos intensos han tenido desgarro de parte del tubo digestivo que conecta su boca con su estómago (esófago)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano, Website: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Alzerta dos por semana
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en el sobre después de CAD. La fecha de caducidad es el último día del mes que se indica.
- Este medicamento no requiere condiciones especiales de conservación.
- No utilizar ningún parche si observa que está dañado o muestra signos de manipulación.
Tras quitarse un parche, dóblelo por la mitad con el lado adhesivo hacia dentro y presione. Tras introducirlo en su sobre, deshágase del parche de tal manera que los niños no puedan manipularlo.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda, pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Alzerta dos por semana
El principio activo es rivastigmina.
Alzerta dos por semana 4,6 mg/24 h parches transdérmicos:
Cada parche transdérmico libera 4,6 mg de rivastigmina en 24 horas. Cada parche transdérmico de 10,8 cm2 contiene 25,92 mg de rivastigmina.
Alzerta dos por semana 9,5 mg/24 h parches transdérmicos:
Cada parche transdérmico libera 9,5 mg de rivastigmina en 24 horas. Cada parche transdérmico de 21,6 cm2 contiene 51,84 mg de rivastigmina.
Los demás componentes del parche transdérmico son:
Lámina externa: lámina de polietileno tereftalato.
Lámina activa: tocoferol, poli[(2-etilhexil)acrilato, vinilacetato (1:1)], copolimero de acrilato de butilo y metacrilato de butilo.
Membrana permeable al fármaco: lámina de polietileno
Lámina adhesiva: poliisobutileno de peso molecular medio, poliisobutileno de peso molecular alto, polibuteno.
Lámina de liberación: lámina de poliéster siliconada.
Tinta de impresión azul.
Aspecto del producto y contenido del envase
Cada parche transdérmico es un parche fino de forma rectangular con los bordes redondeados. El parche es traslúcido y etiquetado con:
- Alzerta dos por semana 4,6 mg/24 h parches transdérmicos:RID-TDS 4.6 mg/24h
- Alzerta dos por semana 9,5 mg/24 h parches transdérmicos:RID-TDS 9.5 mg/24h
Los parches transdérmicos viene sellados y separados en sobres. Los sobres están etiquetados con:
- Alzerta dos por semana 4,6 mg/24 h parches transdérmicos
- Alzerta dos por semana 9,5 mg/24 h parches transdérmicos
Además de cada parche transdérmico, el envase incluye cubiertas adhesivas para la fijación.
Cada cubierta adhesiva es delgada, beige y oval.
Las cubiertas adhesivas vienen selladas y separadas en sobres. Los sobres están etiquetados con: Cubierta adhesiva sin sustancia activa.
Alzerta dos por semana 4,6 mg/24 h parches transdérmicosy Alzerta dos por semana 9,5 mg/24 h parches transdérmicos, se encuentran disponibles en envases que contienen 2, 8, 16 o 24 parches transdérmicos y 2, 8, 16 o 24 cubiertas adhesivas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Esteve Pharmaceuticals, S.A.
Passeig de la Zona Franca, 109
08038 Barcelona
España
Responsable de la fabricación
Luye Pharma AG
Am Windfeld 35
83714 Miesbach
Alemania
Fecha de la última revisión de este prospecto:Noviembre2024
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ALZERTA DOS POR SEMANA 4,6 MG/24 H PARCHES TRANSDERMICOSForma farmacéutica: PARCHE TRANSDERMICO, 13,3 mg/24 hPrincipio activo: RivastigminaFabricante: Esteve Pharmaceuticals S.A.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 4,6 mg/24 hPrincipio activo: RivastigminaFabricante: Esteve Pharmaceuticals S.A.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 9,5 mg/24 hPrincipio activo: RivastigminaFabricante: Esteve Pharmaceuticals S.A.Requiere receta
Médicos online para ALZERTA DOS POR SEMANA 4,6 MG/24 H PARCHES TRANSDERMICOS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ALZERTA DOS POR SEMANA 4,6 MG/24 H PARCHES TRANSDERMICOS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes