ACEOTO PLUS 3 mg/ml + 0,25 mg/ml GOTAS ÓTICAS EN SOLUCIÓN

Cómo usar ACEOTO PLUS 3 mg/ml + 0,25 mg/ml GOTAS ÓTICAS EN SOLUCIÓN
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
Aceoto Plus 3 mg/ml + 0,25 mg/ml gotas óticas en solución
Ciprofloxacino / Acetónido de fluocinolona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas, ya que puede perjudicarles.
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Aceoto Plus y para qué se utiliza
- Qué necesita saber antes de empezar a usar Aceoto Plus
- Cómo usar Aceoto Plus
- Posibles efectos adversos
- Conservación de Aceoto Plus
- Contenido del envase e información adicional
1. Qué es Aceoto Plus y para qué se utiliza
Aceoto Plus es una solución para uso ótico (en el oído).
Contiene:
- Ciprofloxacino, que pertenece al grupo de antibióticos denominados fluoroquinolonas.
Ciprofloxacino actúa eliminando bacterias que causan infecciones,
- y, acetónido de fluocinolona, corticosteroide con propiedades anti-inflamatorias y analgésicas para el tratamiento de la inflamación y el dolor.
Los antibióticos se utilizan para tratar infecciones bacterianas y no sirven para tratar infecciones víricas como la gripe o el catarro. Es importante que siga las instrucciones relativas a la dosis, el intervalo de administración y la duración del tratamiento indicadas por su médico. No guarde ni reutilice este medicamento. Si una vez finalizado el tratamiento le sobra antibiótico, devuélvalo a la farmacia para su correcta eliminación. No debe tirar los medicamentos por el desagüe ni a la basura. |
Aceoto Plus es una solución de gotas para los oídos. Se utiliza en adultos y en niños de 6 meses de edad y mayores para tratar la otitis externa aguda (infección del oído externo) y la otitis media (infección del oído medio) con tubos de timpanostomía (tubos de drenaje) de origen bacteriano.
Debe consultar a un médico si empeora o si no mejora después de finalizar el tratamiento.
2. Qué necesita saber antes de empezar a usar Aceoto Plus
No use Aceoto Plus
- Si es alérgico al ciprofloxacino, a otras quinolonas, a acetónido de fluocinolona o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si usted tiene una infección de oído causada por un virus o un hongo.
Advertencias y precauciones
- Este medicamento sólo se debe aplicar en el oído. No debe ser ingerido, inyectado o inhalado. No se debe aplicar en el ojo.
- Si una vez iniciado el tratamiento se producen síntomas de urticaria (picor) o erupción cutánea o cualquier otro síntoma alérgico (por ejemplo, hinchazón repentina de la cara, la garganta o los párpados, dificultad respiratoria), interrumpa inmediatamente la medicación y acuda a su médico. Las reacciones graves de hipersensibilidad pueden necesitar tratamiento inmediato de urgencia.
- Informe a su médico si los síntomas no mejoran antes de finalizar el tratamiento.
Al igual que con otros antibióticos, las infecciones pueden ser a veces causadas por organismos que no son susceptibles a ciprofloxacino. En caso de dichas infecciones, el tratamiento apropiado debe ser prescrito por su médico.
- Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Uso en niños
Niños: No hay suficiente experiencia clínica sobre el uso de Aceoto Plus en niños menores de 6 meses, por lo que debe consultar con su médico antes de administrar este medicamento a su hijo si tiene esta edad.
Uso de Aceoto Plus con otros medicamentos
Informe a su médico o farmacéutico si está tomando/utilizando, ha tomado/utilizado recientemente o podría tener que tomar/utilizar cualquier otro medicamento, incluso los adquiridos sin receta.
No se recomienda el uso de Aceoto Plus junto con otros medicamentos por vía ótica (a través del oído).
Embarazo, lactancia y fertilidad
No hay estudios adecuados y bien controlados en mujeres embarazadas sobre los efectos teratogénicos de acetónido de fluocinolona.
Si está embarazada o en periodo de lactancia, o cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Se debe tener precaución cuando se administre Aceoto Plus a una mujer lactante ya que no se sabe si Aceoto Plus se excreta a través de la leche materna.
Conducción y uso de máquinas
Dadas las características y vía de administración del medicamento, Aceoto Plus no afecta a la capacidad de conducir vehículos o manejar máquinas peligrosas.
Aceoto Plus contieneParahidroxibenzoato de metilo y Parahidroxibenzoato de propilo
Aceoto Plus puede producir reacciones alérgicas (posiblemente retardadas) porque contiene parahidroxibenzoato de metilo y parahidroxibenzoato de propilo.
3. Cómo usar Aceoto Plus
Aceoto Plus está destinado a ser administrado en el oído solamente (uso ótico). Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada en adultos y niños es de 6 a 8 gotas dos veces al día en el oído afectado durante 7 días.
Sólo administre Aceoto Plus en ambos oídos si su doctor así lo recomienda.
Su médico le indicará la duración del tratamiento con Aceoto Plus. Para asegurarse de que la infección no reaparece, no interrumpa el tratamiento anticipadamente, incluso si nota mejoría en el oído (s).
Instrucciones de administración
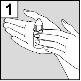
La persona que administre Aceoto Plus debe lavarse previamente las manos.
|
|
|
|
|
|
|
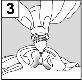
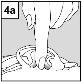
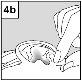
Para los pacientes con infección del oído medio con tubos de drenaje: Mientras el paciente permanece recostado de lado, la persona encargada de administrar Aceoto Plus debe presionar con suavidad el pliegue de piel situado al inicio del canal auditivo (figura 4a) 4 vecescon un movimiento de bombeo. Este hecho permitirá que las gotas atraviesen el tímpano y lleguen al oído medio. Para los pacientes con infeccióndel oído externo: Mientras el paciente permanece recostado de lado, la persona encargada de administrar Aceoto Plus debe tirar con suavidad del lóbulo de la oreja hacia adelante y hacia atrás (figura 4b). Este hecho permitirá que las gotas penetren en el canal auditivo. |
- Mantener la cabeza inclinada hacia el lado del oído afectado durante, aproximadamente, un minuto para dar tiempo al medicamento a entrar en el interior del oído.
- Repetir la operación, en caso necesario, en el otro oído.
Es importante que siga estas instrucciones para tener un buen resultado con este medicamento en su oído. Una vez el medicamento se haya administrado en el oído, mantener la cabeza en posición vertical o mover la cabeza con demasiada rapidez puede hacer que se pierda parte de la medicación administrada porque las gotas puedan caer por la cara y no penetren en la parte interna del canal auditivo.
Conserve el frasco hasta la finalización del tratamiento. No lo guarde para su uso posterior.
Si usa más Aceoto Plus del que debe
No se conocen los síntomas relativos a la sobredosis. En caso de sobredosis o de ingestión accidental, informe a su médico o a su farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Aceoto Plus
No use una dosis doble para compensar las dosis olvidadas. Simplemente continúe con su dosis siguiente.
Si interrumpe el tratamiento con Aceoto Plus
No deje de usar Aceoto Plus sin consultar a su médico o farmacéutico. Es muy importante utilizar estas gotas para los oídos durante el tiempo que el médico le haya indicado, incluso si los síntomas mejoran. Si deja de usar este fármaco antes, la infección puede no desaparecer y los síntomas pueden reaparecer o incluso empeorar. También se puede producir resistencia a los antibióticos.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Aceoto Plus puede producir efectos adversos aunque no todas las personas los sufran.
Si experimenta una reacción alérgica grave y le aparece cualquiera de los siguientes síntomas, interrumpa el tratamiento con este medicamento y consulte con su médico de forma inmediata: hinchazón de manos, pies, tobillos, cara, labios, boca o garganta, dificultad para tragar o respirar, erupciones o ronchas, llagas, úlceras.
Frecuentes: pueden afectar hasta 1 de cada 10 personas
Efectos en el oído:Malestar,dolor,picor.
Efectos adversos generales:Alteración en el sabor.
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
Efectos en el oído: Pitidos, residuo de medicamento, bloqueo del tubo de drenaje del oído, hormigueo, congestión, reducción de la audición, erupción cutánea, enrojecimiento, infección por hongos del oído externo, secreción, inflamación, trastorno de la membrana timpánica, tejido de granulación, otitis media en el otro oído.
Efectos adversos generales:Infección por hongos (Candida), irritabilidad, llanto, mareo, rubefacción, dolor de cabeza, vómitos, fatiga.
Frecuencia no conocida (no puede estimarse la frecuencia a partir de los datos disponibles):Visión borrosa.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Aceoto Plus
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar por debajo de 30ºC.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Este medicamento debe ser desechado al mes de su apertura.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Aceoto Plus
Los principios activos son ciprofloxacino (como hidrocloruro) y acetónido de fluocinolona. Cada mililitro de Aceoto Plus contiene 3 mg de ciprofloxacino (como hidrocloruro) y 0,25 mg de acetónido de fluocinolona.
Los demás componentes son parahidroxibenzoato de metilo (E218), parahidroxibenzoato de propilo (E216), povidona-K-90-F, dietilenglicol monoetil éter, glicereth-26 (compuesto de glicerina y óxido de etileno), ácido clorhídrico (E507)/hidróxido de sodio (E524) y agua purificada.
Aspecto del producto y contenido del envase
Aceoto Plus es una solución acuosa incolora o ligeramente amarilla en forma de gotas para la administración en el oído. Se presenta en frascos de plástico blanco opaco con un gotero. Cada envase contiene 10 ml de solución.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Zambon S.A.U.
Maresme, 5. Pol. Can Bernades-Subirà
08130 - Santa Perpètua de Mogoda
Barcelona (España)
Responsable de la fabricación
Laboratorios Salvat, S.A.
C/ Gall 30-36
08950 - Esplugues de Llobregat
Barcelona (España)
o
Pharmaloop, S.L.
C/Bolivia, 15 – Polig.Industrial Azque
28806 Alcalá de Henares,
Madrid (España)
Este prospecto ha sido aprobado en Noviembre 2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Precio medio en farmacia7.91 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ACEOTO PLUS 3 mg/ml + 0,25 mg/ml GOTAS ÓTICAS EN SOLUCIÓNForma farmacéutica: LÍQUIDO OTICO, 3 mg/ml + 0,25 mg/mlPrincipio activo: fluocinolone acetonide and antiinfectivesFabricante: Laboratorios Salvat S.A.Requiere recetaForma farmacéutica: LÍQUIDO OTICO, 3mg ciprofloxacino hcl.;0,25mg fluocinolona acetonPrincipio activo: fluocinolone acetonide and antiinfectivesFabricante: Laboratorios Salvat S.A.Requiere recetaForma farmacéutica: LÍQUIDO OTICO, 0,25 - 3 mg/mlPrincipio activo: fluocinolone acetonide and antiinfectivesFabricante: Industrial Farmaceutica Cantabria S.A.Requiere receta
Médicos online para ACEOTO PLUS 3 mg/ml + 0,25 mg/ml GOTAS ÓTICAS EN SOLUCIÓN
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ACEOTO PLUS 3 mg/ml + 0,25 mg/ml GOTAS ÓTICAS EN SOLUCIÓN, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes