
ZOFRAN ZYDIS 4 mg LIOFILIZADO ORAL

Cómo usar ZOFRAN ZYDIS 4 mg LIOFILIZADO ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Zofran Zydis 4 mg liofilizado oral
ondansetrón
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Zofran Zydis y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Zofran Zydis
- Cómo tomar Zofran Zydis
- Posibles efectos adversos
- Conservación de Zofran Zydis
- Contenido del envase e información adicional
1. Qué es Zofran Zydis y para qué se utiliza
Zofran Zydis pertenece al grupo de medicamentos denominados antieméticos. Ondansentrón es un antagonista del receptor 5HT3. Actúa inhibiendo los receptores 5HT3 en las neuronas ubicadas en el sistema nervioso central y periférico.
Ondansetrón se utiliza para:
- prevenir las náuseas y los vómitos causados por:
- la quimioterapia en el tratamiento del cáncer en adultos y niños mayores
de 6 meses de edad.
- la radioterapia en el tratamiento del cáncer en adultos.
- prevenir las náuseas y vómitos postoperatorios en adultos.
2. Qué necesita saber antes de empezar a tomar Zofran Zydis
No tome Zofran Zydis
- Si es alérgico (hipersensible) a ondansetrón o a cualquiera de los componentes de Zofran Zydis (incluidos en la sección 6).
- Si tiene o ha tenido alguna reacciónalérgica(hipersensibilidad) con otros medicamentos para las náuseas o vómitos (por ejemplo, granisetrón o dolasetrón).
- Si está tomando apomorfina (medicamento usado para tratar la enfermedad de Parkinson).
→ Si considera que esto le aplica, no tome Zofran Zydis y consulte a su médico.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Zofran Zydis.
- Si padece un bloqueo en el intestino o si sufre de estreñimiento grave. Zofran Zydis puede aumentar el bloqueo o el estreñimiento.
- Si alguna vez ha tenido problemas de corazón, incluyendo ritmo cardiaco irregular (arritmia).
- Si está siendo sometido a una operación de amígdalas.
- Si tiene problemas de hígado.
Si le van a realizar alguna prueba diagnóstica (incluidos análisis de sangre, orina, pruebas cutáneas que utilizan alérgenos, etc.) comunique a su médico que está tomando este medicamento, ya que puede alterar los resultados.
Otros medicamentos y Zofran Zydis
Informe a su médico o farmacéutico si está tomando o ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
En particular, es importante informar al médico si está tomando cualquiera de los siguientes medicamentos, ya que puede ser necesario interrumpir el tratamiento o ajustar la dosis de alguno de ellos:
- rifampicina(antibiótico usado para tratar infecciones tales como la tuberculosis).
- tramadoly buprenorfina(medicamentos usados para tratar el dolor grave).
- fenitoínao carbamacepina(medicamentos utilizados para tratar la epilepsia).
- medicamentos utilizados para tratar problemas del corazóncomo alteraciones en los latidos (antiarrítmicos) y/o para tratar la tensión alta(betabloqueantes).
- haloperidolo metadona(medicamentos que pueden afectar al corazón).
- antraciclinasy trastuzumab(medicamentos utilizados para tratar el cáncer).
- fluoxetina, paroxetina, sertralina, fluvoxamina, citalopram, escitalopram(Inhibidores selecivos de la recaptación de serotonina, empleados para tratar la depresión y/o ansiedad)
- venlafaxina, duloxetina(Inhibidores de la recaptación de serotonina y noradrenalina, empleados para tratar la depresión y/o ansiedad).
Informe a su médico o farmacéuticosi está tomando alguno de estos medicamentos.
Informe a su médico o farmacéutico inmediatamente si nota alguno de estos síntomas durante o después del tratamiento
- si nota un dolor repentino u opresión en el pecho (isquemia miocárdica)
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Zofran Zydis no debe utilizarse durante el primer trimestre del embarazo. Esto se debe a que Zofran Zydis puede aumentar ligeramente el riesgo de que un bebé nazca con labio leporino y/o fisura del palatina (aberturas o hendiduras en el labio superior o en el paladar). Si ya está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar Zofran Zydis, ya que Zofran Zydis puede causar daño en el feto. Si es una mujer en edad fértil, se le recomienda utilizar un método anticonceptivo efectivo.
Si usted es una mujer en edad fértil, su médico o enfermero deberá comprobar si está embarazada y le realizará una prueba de embarazo antes de comenzar el tratamiento con Zofran Zydis.
Las mujeres en edad fértil deben usar un método anticonceptivo eficaz durante el tratamiento con Zofran Zydis. Consulte a su médico acerca de las opciones de métodos anticonceptivos.
Si se queda embarazada durante el tratamiento con Zofran Zydis, informe a su médico.
No se recomienda dar el pecho durante el tratamiento con Zofran Zydis, los ingredientes de Zofran Zydis (ondansetrón) pueden pasar a la leche materna y pueden afectar a su bebé.
Conducción y uso de máquinas
Es poco probable que Zofran Zydis pueda afectar la capacidad para conducir y utilizar máquinas.
Zofran Zydis contiene aspartamo (E951), parahidroxibenzoato de metilo sódico (E219), parahidroxibenzoato de propilo sódico y alcohol bencílico (E1519)
Este medicamento contiene 0,625 mg de aspartamo por liofilizado oral. El aspartamo contiene una fuente de fenilalanina que puede ser perjudicial en caso de padecer fenilcetonuria (FCN), una enfermedad genética rara en la que la fenilalanina se acumula debido a que el organismo no es capaz de eliminarla correctamente.
Este medicamento puede provocar reacciones alérgicas (posiblemente retardadas) porque contiene parahidroxibenzoato de metilo sódico y parahidroxibenzoato de propilo sódico.
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por liofilizado oral, esto es esencialmente "exento de sodio".
Este medicamento contiene 0,000025 mg de alcohol bencílico en cada liofilizado oral.
El alcohol bencílico puede provocar reacciones alérgicas. El alcohol bencílico se ha relacionado con el riesgo de efectos adversos graves que incluyen problemas respiratorios (“síndrome de jadeo”) en niños. No administre este medicamento a su recién nacido (hasta de 4 semanas de edad) a menos que se lo haya recomendado su médico. Este producto no se debe utilizar durante más de una semana en niños menores de 3 años de edad a menos que se lo indique su médico o farmacéutico. Consulte a su médico o farmacéutico si está embarazada o en periodo de lactancia o si tiene enfermedades de hígado o riñón. Esto es debido a que se pueden acumular grandes cantidades de alcohol bencílico en su organismo y provocar efectos adversos (acidosis metabólica).
3. Cómo tomar Zofran Zydis
Su médico le dirá exactamente la cantidad que debe tomar de Zofran Zydis.En caso de duda, consulte de nuevo a su médico o farmacéutico.
Recuerde tomar su medicamento. Su médico le indicará la duración de su tratamiento con Zofran Zydis. No suspenda el tratamiento antes.
Náuseas y vómitos causados por quimioterapia o radioterapia
A
Dos liofilizados orales (8 mg) 1-2 horas antes del tratamiento médico potencialmente causante de las náuseas y los vómitos, y a continuación 2 liofilizados orales (8 mg) 12 horas más tarde.
Para prevenir las náuseas y vómitos en días posteriores, continúe tomando 2 liofilizados orales (8 mg) cada 12 horas durante cinco días.
Náuseas y vómitos causados por quimioterapia
Niños mayores de 6meses de edad y adolescentes
El médico decidirá la dosis en función del peso o de la superficie corporal de su hijo.
Normalmente, doce horas después de la quimioterapia su hijo recibirá ondansetrón por vía oral. La dosis habitual es de 4 mg dos veces al día y puede continuar durante un periodo de hasta 5 días.
Náuseas y vómitos postoperatorios
Adultos
Para prevenir náuseas y vómitos después de una intervención quirúrgica, 4 liofilizados orales (16 mg) 1 hora antes de la anestesia.
Niños
No se dispone de datos sobre la administración por vía oral de Zofran Zydis en la prevención de náuseas y vómitos postoperatorios en niños.
Ajustes de la dosis
Pacientes con insuficiencia hepática
En los pacientes con problemas hepáticos, la dosis debe ajustarse a un máximo de 8 mg diarios de Zofran Zydis.
Pacientes de edad avanzada, pacientes con insuficiencia renal o metabolizadores lentos de esparteína/debrisoquina
No es necesario modificar la dosis diaria o la frecuencia de la dosis o la vía de administración.
Duración del tratamiento
Su médico decidirá la duración de su tratamiento con Zofran Zydis. No suspenda el tratamiento antes.
Si estima que la acción de Zofran Zydis es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Zofran Zydis es un tipo de liofilizado oral que, al depositarse en la parte superior de la lengua, desaparece muy rápidamente.
Separar la lámina superior de aluminio de un alvéolo ysacar cuidadosamentela unidad liofilizada oral de Zofran Zydis.
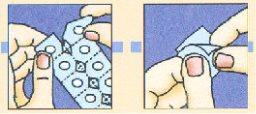
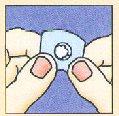 No intentar sacar el liofilizado oral de Zofran Zydis rompiendo la lámina superior de aluminio ya que la unidad liofilizada oral es frágil y se rompería dentro
No intentar sacar el liofilizado oral de Zofran Zydis rompiendo la lámina superior de aluminio ya que la unidad liofilizada oral es frágil y se rompería dentro
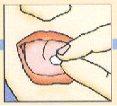
Colocar el liofilizado oral en la parte superior de la lengua; desaparecerá muy rápidamente; a continuación ingerirlo de la forma habitual.
Si toma más Zofran Zydis del que debe
En caso de sobredosificación, los síntomas que pueden aparecer son: problemas de visión, presión baja de la sangre (lo que puede causar mareos o desmayos) y palpitaciones (latido irregular del corazón).
Si usted o su hijo toma más Zofran Zydis del que debe, hable con un médico o vaya inmediatamente al hospital más cercano o llame al Servicio de Información Toxicológica, teléfono: 91 5620420, indicando el medicamento y la cantidad ingerida. Lleve el medicamento consigo.
Si olvidó tomar Zofran Zydis
No tome una dosis doble para compensar las dosis olvidadas. No aumentar ni disminuir la dosis sin autorización del médico.
En caso de que olvide tomar una dosis de Zofran Zydis y presente molestias o vómitos, tome otra dosis tan pronto como sea posible. Luego, continúe tomando el medicamento como se le ha indicado.
En caso de que olvide tomar una dosis de Zofran Zydis y no presente molestias, espere a la siguiente toma y continúe tomando el medicamento como se le ha indicado.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos muy frecuentes
Pueden afectar a más de 1 de cada 10 pacientes
- dolor de cabeza.
Efectos adversos frecuentes
Pueden afectar hasta 1 de cada 10 pacientes
- sensación de calor o rubor,
- estreñimiento.
Efectos adversos poco frecuentes
Pueden afectar hasta 1 de cada 100 pacientes
- movimientos giratorios ascendentes de los ojos, rigidez muscular anormal, movimientos del cuerpo, temblor,
- convulsiones,
- latidos lentos o irregulares del corazón,
- presión sanguínea más baja de lo normal (hipotensión),
- hipo,
- aumento de los niveles en los resultados de pruebas sanguíneas de comprobación del funcionamiento del hígado.
Efectos adversos raros
Pueden afectar a menos de 1 de cada 1.000 pacientes
- aparición repentina de pitidos y dolor u opresión en el pecho,
- hinchazón de párpados, cara, labios, boca o lengua,
- erupción en la piel o urticaria en cualquier parte del cuerpo,
- alteración del ritmo cardiaco (en ocasiones pueda causar una pérdida repentina del conocimiento),
- visión borrosa.
Si experimenta alguno de estos síntomas, deje de tomar el medicamento inmediatamente y avise a su médico.
Efectos adversos muy raros
Pueden afectar a menos de 1 de cada 10.000 pacientes
- ceguera transitoria, que normalmente se resuelve en 20 minutos,
- alteraciones en el electrocardiograma,
- erupción extensa en la piel con ampollas y descamación, que afecta una gran
parte la superficie corporal (necrolisis epidérmica tóxica),
- Informe inmediatamente a su médico si presenta alguno de estos síntomas.
Efectos adversos de frecuencia no conocida
No puede estimarse su frecuencia a partir de los datos disponibles
. isquemia miocárdica
- aparición repentina de dolor repentino en el pecho u,
opresión en el pecho (isquemia miocárdica).
→ Si experimenta alguno de estos síntomas, deje de tomar el medicamento inmediatamente y avise a su médico.
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Zofran Zydis
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 30 ºC.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE E de la farmacia . Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Zofran Zydis 4 mg liofilizado oral
El principio activo es ondansetrón. Cada liofilizado oral contiene 4 mg de ondansetrón.
Los demás componentes (excipientes) son: gelatina, manitol (E421), aspartamo (E951), parahidroxibenzoato de metilo sódico (E219), parahidroxibenzoato de propilo sódico y sabor fresa (contiene aroma de fresa, propilenglicol (E1520), alcohol bencílico (E1519) y sodio).
Aspecto del producto y contenido del envase
Zofran Zydis 4 mg se presenta en forma de liofilizados orales redondos, de color blanco, plano-convexo y de rápida dispersión. Cada envase contiene 10 o 500 liofilizados orales.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
BEXAL FARMACÉUTICA, S.A.
Centro Empresarial Parque Norte
Edificio Roble
C/ Serrano Galvache, 56
28033 Madrid
España
Responsable de la fabricación
Aspen Bad Oldesloe GmbH
Industriestrasse 32-36
23843 Bad Oldesloe
Alemania
o
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona – España
o
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Alemania
o
LEK Pharmaceuticals d.d.,
Verovškova ulica 57,
1526 Ljubljana,
Eslovenia
Fecha de la última revisión de este prospecto:julio 2024.
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http: //www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ZOFRAN ZYDIS 4 mg LIOFILIZADO ORALForma farmacéutica: INYECTABLE, 2 MG/MLPrincipio activo: OndansetronFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: COMPRIMIDO BUCODISPERSABLE/LIOTAB, 4 MGPrincipio activo: OndansetronFabricante: Aristo Pharma Iberia S.L.Requiere recetaForma farmacéutica: COMPRIMIDO BUCODISPERSABLE/LIOTAB, 8 MGPrincipio activo: OndansetronFabricante: Aristo Pharma Iberia S.L.Requiere receta
Médicos online para ZOFRAN ZYDIS 4 mg LIOFILIZADO ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ZOFRAN ZYDIS 4 mg LIOFILIZADO ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















