VYVGART 1.000 MG SOLUCION INYECTABLE

Cómo usar VYVGART 1.000 MG SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Vyvgart 1 000 mg solución inyectable
efgartigimod alfa
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Vyvgart y para qué se utiliza
- Qué necesita saber antes de empezar a usar Vyvgart
- Cómo usar Vyvgart
- Posibles efectos adversos
- Conservación de Vyvgart
- Contenido del envase e información adicional
1. Qué es Vyvgart y para qué se utiliza
Qué es Vyvgart
Vyvgart contiene el principio activo efgartigimod alfa. Efgartigimod alfa se une a una proteína del organismo denominada receptor neonatal para el Fc (FcRn) y la bloquea. Al bloquear el FcRn, efgartigimod alfa reduce el nivel de autoanticuerpos frente a inmunoglobulina G (IgG), que son proteínas del sistema inmunitario que atacan por error partes del organismo de una persona.
Para qué se utiliza Vyvgart
Vyvgart se utiliza junto con el tratamiento de referencia para tratar a adultos con miastenia gravis generalizada (MGG), una enfermedad autoinmune que provoca debilidad muscular. La MGG puede afectar a varios grupos musculares de todo el cuerpo. La enfermedad también puede provocar falta de aire, fatiga extrema y dificultad para tragar.
En los pacientes con MGG, los autoanticuerpos frente a IgG atacan y dañan a unas proteínas de los nervios denominadas receptores de acetilcolina. A causa de este daño, los nervios no son capaces de contraer los músculos normalmente, lo que provoca debilidad muscular y dificultad para moverse. Al unirse a la proteína FcRn y reducir los niveles de autoanticuerpos, Vyvgart puede mejorar la capacidad de contracción de los músculos y reducir los síntomas de la enfermedad y su repercusión en las actividades diarias.
2. Qué necesita saber antes de empezar a usar Vyvgart
No use Vyvgart
- si es alérgico a efgartigimod alfa o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Vyvgart.
Clase V de MGFA
El médico no puede recetarle este medicamento si está conectado a un respirador debido a la debilidad muscular por MGG (crisis miasténica).
Infecciones
El tratamiento con Vyvgart puede reducir su resistencia natural a las infecciones, por lo que debe informar a su médico si tiene alguna infección antes de empezar a usar Vyvgart.
Reacciones a la inyección y reacciones alérgicas
Vyvgart contiene una proteína que puede provocar en algunas personas reacciones como erupción o picazón. Vyvgart puede causar una reacción anafiláctica (reacción alérgica grave). Si experimenta reacciones alérgicas como hinchazón de la cara, labios, garganta o lengua que dificulte tragar o respirar, falta de aire, sensación de pérdida de consciencia o erupción cutánea durante o después de la inyección, informe a su médico de inmediato.
Inmunizaciones (vacunas)
Informe a su médico si se le ha administrado alguna vacuna en las últimas 4 semanas, o si tiene previsto vacunarse en un futuro próximo.
Niños y adolescentes
No administre este medicamento a niños menores de 18 años, ya que no se ha establecido la seguridad y eficacia de Vyvgart en esta población.
Pacientes de edad avanzada
No son necesarias precauciones especiales para el tratamiento de pacientes mayores de 65 años.
Otros medicamentos y Vyvgart
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
No se espera que Vyvgart influya en la capacidad de conducir o utilizar máquinas.
Vyvgart contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por vial; esto es, esencialmente “exento de sodio”.
Vyvgart contiene polisorbato
Este medicamento contiene 2,7 mg de polisorbato 20 en cada vial, equivalente a 0,4 mg/ml. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene alguna alergia conocida.
3. Cómo usar Vyvgart
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Qué dosis de Vyvgart recibirá y con qué frecuencia
La dosis recomendada es de 1 000 mg administrados en ciclos de una inyección por semana durante 4 semanas. Su médico decidirá cuándo son necesarios más ciclos de tratamiento.
Si ya está en tratamiento con Vyvgart por vía intravenosa y desea cambiar a Vyvgart por vía subcutánea, deberá recibir la inyección subcutánea en lugar de su perfusión intravenosa al inicio del siguiente ciclo de tratamiento.
Inyección de Vyvgart
Vyvgart se administra mediante una inyección debajo de la piel (por vía subcutánea). Usted y su médico deben decidir si, tras una formación adecuada, usted o su cuidador pueden inyectar Vyvgart. La primera autoinyección se debe realizar delante de su profesional sanitario. Es importante que no intente inyectar Vyvgart antes de haber recibido formación por parte de un profesional sanitario.
Si usted o su cuidador inyectan Vyvgart, usted o su cuidador deben leer atentamente y seguir las Instrucciones de administración que figuran al final de este prospecto (ver “Instrucciones de uso importantes”). Hable con su médico, farmacéutico o enfermero si tiene alguna duda sobre cómo administrarse una inyección.
Si usa más Vyvgart del que debe
Dado que Vyvgart se administra en un vial de un solo uso, es poco probable que reciba demasiada cantidad. No obstante, si está preocupado, póngase en contacto con su médico, farmacéutico o enfermero para que le aconseje.
Si se perdió u olvidó una cita para recibir Vyvgart
Lleve un registro de su próxima dosis. Es importante que use Vyvgart exactamente como se lo haya recetado su médico.
- Si olvidó tomar su dosis en los tres días siguientes a la fecha en que debía tomarla, tome su dosis tan pronto como lo recuerde y luego siga con la pauta posológica original.
- Si olvidó tomar su dosis durante más de tres días, pregunte a su médico cuándo debe tomar la siguiente dosis.
- Si olvidó una cita, póngase en contacto con su médico inmediatamente para que le aconseje.
No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Vyvgart
La interrupción o el cese del tratamiento con Vyvgart puede provocar la reaparición de sus síntomas de MGG. Consulte con su médico antes de interrumpir el tratamiento con Vyvgart. Su médico le explicará los posibles efectos adversos y riesgos. Su médico también querrá supervisarle estrechamente.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Su médico le explicará los posibles efectos adversos y los riesgos y beneficios de Vyvgart antes del tratamiento.
Informe a su médico de inmediato si nota:
Signos de una reacción alérgica grave (reacción anafiláctica) como hinchazón de la cara, labios, garganta o lengua que dificulte tragar o respirar, falta de aire, sensación de pérdida de conocimiento o erupción cutánea durante o después de la inyección.
Si no está seguro de cuáles son los efectos adversos que se indican a continuación, pida a su médico que se los explique.
Muy frecuentes(pueden afectar a más de 1 de cada 10 pacientes)
- infecciones de la nariz y la garganta (vías respiratorias altas)
- reacciones en la zona de inyección, que pueden incluir enrojecimiento, picor, dolor. Estas reacciones en la zona de inyección suelen ser de leves a moderadas y suelen aparecer un día después de la inyección.
Frecuentes(pueden afectar hasta a 1 de cada 10 pacientes)
- dolor o sensación de ardor al orinar, que puede ser un signo de infección urinaria
- inflamación de las vías respiratorias en los pulmones (bronquitis)
- dolor muscular (mialgia)
- náuseas.
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- reacciones alérgicas durante o después de la inyección.
- hinchazón de la cara, labios, garganta o lengua que dificulte tragar o respirar, falta de aire.
- piel pálida, pulso débil y rápido, o sensación de pérdida de consciencia.
- erupción repentina, picor, o habones.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Vyvgart
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 °C y 8 °C). No congelar.
En caso necesario, los viales sin abrir pueden conservarse a temperatura ambiente (hasta 30 °C) durante un máximo de 3 días. Después de la conservación a temperatura ambiente, los viales sin abrir pueden devolverse a la nevera. El tiempo total fuera de la nevera y a temperatura ambiente no debe superar los 3 días.
Conservar en el embalaje original para protegerlo de la luz.
No utilice este medicamento si observa partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Vyvgart
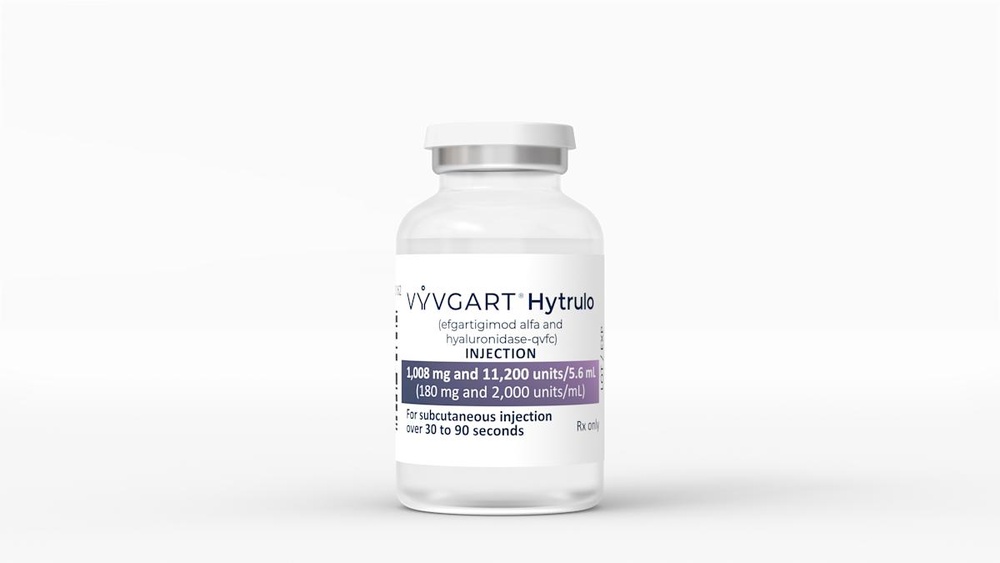
- El principio activo es efgartigimod alfa. Cada vial contiene 1 000 mg de efgartigimod alfa en 5,6 ml. Cada ml contiene 180 mg de efgartigimod alfa.
- Los demás componentes son: hialuronidasa humana recombinante (rHuPH20), L‑histidina, clorhidrato de L-histidina monohidrato, L-metionina, polisorbato 20, cloruro de sodio, sacarosa, agua para preparaciones inyectables. Ver sección 2 “Vyvgart contiene sodio”.
Aspecto del producto y contenido del envase
Vyvgart es una solución lista para usar, ligeramente amarilla, transparente a ligeramente turbia, que se presenta como solución inyectable subcutánea.
Titular de la autorización de comercialización y responsable de la fabricación
argenx BV
Industriepark-Zwijnaarde 7
9052 Gent
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien/Eesti argenx BV Tél/Tel: +32 (0) 93969394/+32 (0) 800 54477 | Lietuva argenx BV Tel: 8 800 80 052 |
| Luxembourg/Luxemburg argenx BV Tel: 800 25 233 |
Ceská republika argenx BV Tel: 800 040 854 | Magyarország argenx BV Tel.: (80) 088 578 |
Danmark argenx BV Tlf.: 80 25 41 88 | Malta argenx BV Tel: 8006 5101 |
Deutschland argenx Germany GmbH Tel: 08001803963 | Nederland argenx BV Tel: 0800 0232882 |
Ελλ?δα Medison Pharma Greece Single Member Societe Anonyme Τηλ: +30 210 0100 188 | Norge argenx BV Tlf: 800 62 225 |
España argenx Spain S.L. Tel: 900 876 188 | Österreich argenx BV Tel: 0800 017936 |
France argenx France SAS Tél: +33 (0) 188898992 | Polska argenx BV Tel.: 800 005 155 |
Hrvatska argenx BV Tel: 0800 806 524 | Portugal argenx BV Tel: 800 180 844 |
Ireland/United Kingdom (Northern Ireland) argenx BV Tel: 1800 851 868 | România argenx BV Tel: 0800 360 912 |
Ísland argenx BV Sími: 800 4422 | Slovenija argenx BV Tel: 080 688955 |
Italia argenx Italia s.r.l Tel: 800729052 | Slovenská republika argenx BV Tel: 0800 002 646 |
Κ?προς argenx BV Τηλ: 80 077122 | Suomi/Finland argenx BV Puh/Tel: 0800 412838 |
Latvija argenx BV Tel: 80 205 267 | Sverige argenx BV Tel: 020‑12 74 56 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
--------------------------------------------------------------------------------------------------------------------Instrucciones de uso importantes
Vyvgart 1 000 mg solución inyectable
efgartigimod alfa
Vía subcutánea
Lea y comprenda estas instrucciones de uso antes de aplicar la inyección de Vyvgart.
Si usted o su cuidador están dispuestos a administrar Vyvgart, su profesional sanitario les indicará cómo inyectarlo. Su profesional sanitario debe enseñarles a usted o a su cuidador cómo preparar y aplicar la inyección de Vyvgart correctamente antes de utilizarlo por primera vez. Se requiere una demostración de autoadministración adecuada bajo la supervisión de un profesional sanitario. Es importante que no intente inyectarse el medicamento hasta que haya recibido formación y usted o su cuidador estén seguros de que saben cómo utilizar Vyvgart. Pregunte a su profesional sanitario si tiene alguna duda.
Información importante que debe conocer antes aplicar una inyección subcutánea de Vyvgart.
- Solo por vía subcutánea.
- El vial es de un solo uso. Noguarde los viales, aunque no estén vacíos.
- Noutilice un vial si observa una turbidez inusual o partículas visibles. El medicamento debe tener un aspecto ligeramente amarillo, de transparente a ligeramente turbio.
- Noagite el vial durante la manipulación.
- Noutilice viales deteriorados o sin tapa protectora. Notifique y devuelva a la farmacia los viales deteriorados o sin tapa.
Conservación de Vyvgart
- Conservar en nevera (entre 2 ºC y 8 ºC).
- Nocongelar.
- En caso necesario, los viales sin abrir pueden conservarse a temperatura ambiente (hasta 30 °C) durante un máximo de 3 días. Después de la conservación a temperatura ambiente, los viales sin abrir pueden devolverse a la nevera. El tiempo total fuera de la nevera y a temperatura ambiente no debe superar los 3 días.
- Conservar en el embalaje original para protegerlo de la luz.
- Mantener este medicamento fuera de la vista y del alcance de los niños.
| |
1 vial que contiene Vyvgart |
|
Prospecto e instrucciones de uso de Vyvgart |
|
Material adicional no incluido Conserve el material adicional a temperatura ambiente en un lugar seco | |
Toallitas con alcohol |
|
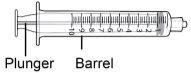
Jeringa de 10 ml |
|
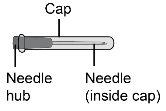
Aguja de transferencia de calibre 18, ≥ 38 mm de longitud |
|
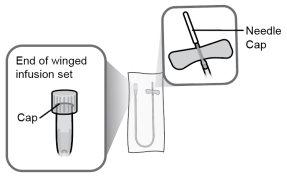
Microperfusor de calibre 25, tubo de 30 cm, volumen máximo de cebado de 0,4 ml |
|
Gasa estéril |
|
Apósito adhesivo |
|
Contenedor para objetos cortopunzantes |
|
Preparación del material
Paso1 Sacar la caja del vial de la nevera. |
|
Paso2 Sacar el vial de la caja y comprobar que:
Si no se cumple alguna de las condiciones anteriores, noadministre la inyección e informe a la farmacia. |
|
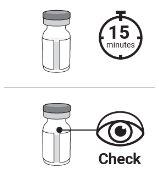
Paso3 Esperar al menos 15 minutos para que el vial se atempere de forma natural hasta alcanzar la temperatura ambiente. Comprobar si el medicamento del vial es ligeramente amarillo, de transparente a ligeramente turbio y no contiene partículas visibles.
|
|
Paso4 Reunir el material adicional siguiente:
| |
Paso5 5a.Limpiar la zona de trabajo. 5b.Lavarse las manos con jabón y secarlas bien. |
|
Preparación de la jeringa | |
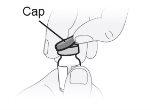
Paso6 Retirar la tapa extraíble protectora de plástico del vial. El precinto de aluminio debe permanecer en su sitio. |
|
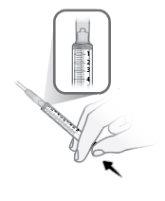
Paso7 Limpiar el tapón de goma con una toallita con alcohol nueva. Dejar secar al aire de forma natural durante al menos 30 segundos. Nosoplar el tapón de goma. |
|
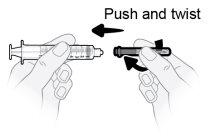
Paso8 Desenvolver la jeringa y la aguja de transferencia. Introducir la aguja de transferencia en la jeringa y girarla en el sentido de las agujas del reloj hasta que la aguja quede firmemente acoplada a la jeringa. Notocar ni la punta de la jeringa ni la parte inferior de la aguja para evitar gérmenes y riesgo de infección. |
|
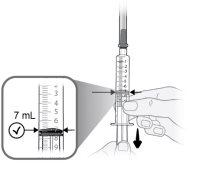
Paso9 Tirar lentamente del émbolo e introducir hasta 7 ml de aire en la jeringa. |
|
Paso10 10a.Sujetar la jeringa por el cono donde la aguja se acopla a la jeringa. 10b.Sujetar el capuchón de la aguja de transferencia y tirar con cuidado para retirarlo del cuerpo. 10c.Colocar el capuchón de la aguja de transferencia hacia abajo sobre una superficie limpia y plana.
|
|
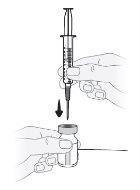
Paso11 Mantener el vial en posición vertical sobre una superficie plana e introducir la aguja de transferencia a través del centro del tapón de goma desinfectado. Noperforar el tapón de goma del vial más de una vez para evitar fugas. |
|
Paso12 Dar la vuelta al vial manteniendo la aguja de transferencia introducida en él. |
|
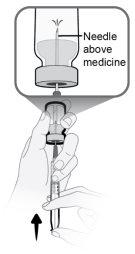
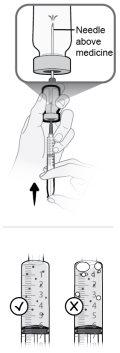
Paso13 13a.Asegurarse de que la aguja de transferencia del vial apunte hacia arriba con la punta de la aguja por encima de la solución de medicamento. 13b.Empujar suavemente el émbolo para inyectar todo el aire de la jeringa en el espacio vacío sobre la solución de medicamento en el vial. 13c.Mantener el dedo presionado sobre el émbolo de la jeringa. Noinyectar aire en la solución de medicamento, ya que se podrían crear burbujas de aire o espuma. |
|
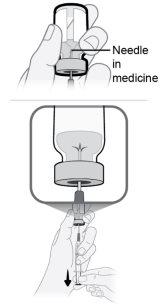
Paso14 Llenar la jeringa como se indica a continuación: 14a.Mantener el dedo presionado sobre el émbolo de la jeringa y deslizar la punta de la aguja de transferencia dentro de la solución de medicamento en el cuello del vial (cerca de la tapa del vial) de forma que la punta de la aguja quede completamente cubierta en la solución. 14b.Retirar lentamente el émbolo, manteniendo la punta de la aguja de transferencia en la solución para evitar burbujas de aire y espuma en la jeringa. Llenar la jeringa con todo el contenido del vial. |
|
Paso15 Eliminar las burbujas de aire grandes, si las hay. 15a.Mantener la aguja de transferencia en el vial y comprobar si hay burbujas de aire grandes en la jeringa. 15b.Eliminar las burbujas de aire grandes dando golpecitos suaves en la jeringa con los dedos hasta que las burbujas suban a la parte superior de la jeringa. 15c.Mover la punta de la aguja de transferencia por encima de la solución de medicamento y empujar lentamente el émbolo hacia arriba para expulsar las burbujas de aire de la jeringa. 15d.Para eliminar cualquier resto de solución de medicamento del vial, volver a introducir la punta de la aguja de transferencia en la solución y tirar lentamente del émbolo hasta tener todo el contenido del vial en la jeringa. 15e.Repetir los pasos anteriores hasta que se hayan eliminado las burbujas de aire grandes. Si no se puede extraer todo el líquido del vial, ponerlo en posición vertical para llegar a la cantidad restante. |
|
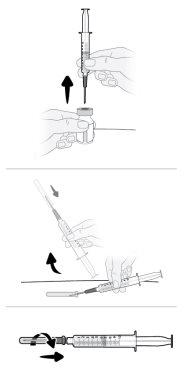
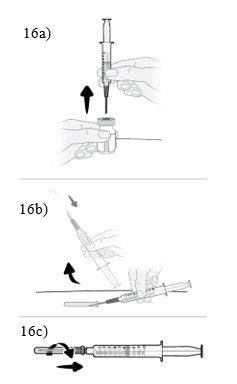
Paso16 16a.Girar el vial en posición vertical y retirar la jeringa y la aguja de transferencia del vial. 16b.Con una mano, deslizar la aguja de transferencia en el capuchón y levantar para cubrir la aguja. 16c.Una vez cubierta la aguja de transferencia, enroscar el capuchón en la jeringa para fijarlo por completo. |
|
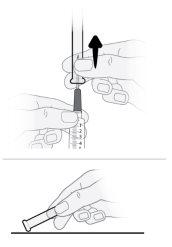
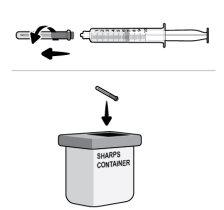
Paso17 17a.Tirar suavemente de la aguja de transferencia y girarla en sentido contrario a las agujas del reloj para retirarla de la jeringa. 17b.Tirar (desechar) la aguja de transferencia en el contenedor para objetos cortopunzantes. |
|
Preparación de la inyección de Vyvgart
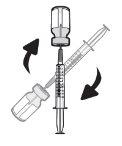
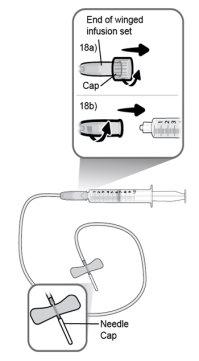
Paso18 18a.Retirar la tapa del extremo del microperfusor. 18b.Empujar y girar suavemente el extremo del microperfusor en el sentido de las agujas del reloj sobre la jeringa hasta que quede firmemente acoplado. La configuración final de la jeringa debe parecerse a la figura de la derecha.
|
|
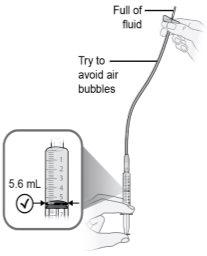
Paso19 19a.Llenar el tubo del microperfusor presionando suavemente el émbolo de la jeringa hasta que esté en la marca de 5,6 ml. Debe verse un poco de líquido en el extremo de la aguja. 19b.Colocar la jeringa y el microperfusor acoplado sobre una superficie limpia y plana. Nolimpiar el exceso de solución de medicamento expulsada del equipo de perfusión durante el llenado del tubo. |
|
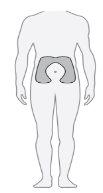
Paso20 Elegir el lugar de inyección
Elegir un lugar de inyección diferente cada vez que se inyecte (rotar el lugar) para disminuir las molestias. Nota: Noinyectar en zonas donde la piel esté enrojecida, amoratada, sensible, dura o en zonas donde haya lunares o cicatrices. |
|
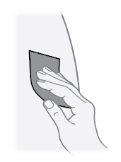
Paso21 Desinfectar el lugar de inyección con una toallita con alcohol nueva. Realizar movimientos circulares y limpiar desde el interior hacia el exterior. Dejar secar la zona al aire durante al menos 30 segundos. Notocar el lugar de inyección después de desinfectarlo. |
|
Inyección de Vyvgart
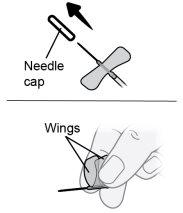
Paso22 22a.Retirar con cuidado el capuchón de la aguja del microperfusor. 22b.Doblar las alas del equipo de perfusión hacia arriba y sujetarlas entre los dedos pulgar e índice, con la aguja por debajo de las alas. Nota: Para evitar infecciones, asegúrese de que la aguja no entre en contacto con nada antes de la inserción cutánea. |
|
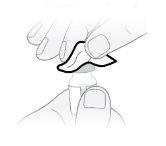
Paso23 Con la mano libre, pellizcar un pliegue de piel alrededor del lugar de inyección desinfectado y levantarlo hacia arriba. Coger suficiente piel para crear una “carpa” en la que introducir la aguja. Nosujetar la piel con demasiada fuerza para evitar que se formen hematomas. |
|
Paso24 Introducir la aguja en el centro de la zona de piel pellizcada en un ángulo de unos 45 grados. Nota: La aguja debe introducirse suavemente en la piel. Si nota resistencia, puede tirar ligeramente de la aguja hacia atrás. |
|
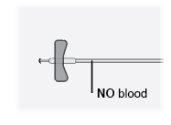
Paso25 Comprobar el equipo de perfusión. Asegurarse de que no haya sangre. Importante: Si ve sangre, tire ligeramente de la aguja hacia atrás sin retirarla de la piel. |
|
Paso26 Administrar la inyección empujando el émbolo de la jeringa con una presión constante hasta que no quede medicamento en la jeringa. Esto corresponde a la inyección de la dosis recomendada de 5,6 ml. La inyección suele durar entre 30 y 90 segundos. Nota:
|
|
Paso27 27a.Una vez inyectada toda la solución, retirar la aguja de la piel. 27b.Cubrir el lugar de inyección con un apósito estéril, como un apósito adhesivo. Nota: Si se observa una pequeña gota de sangre después de retirar la aguja, nose preocupe. Esto puede ocurrir si la aguja pincha la piel durante la extracción. Limpie la sangre con una gasa estéril y presione con suavidad. No deberían producirse más hemorragias. Aplique un apósito estéril para cubrir la zona. |
|
Eliminación de Vyvgart
Paso28 Tirar (desechar) el microperfusor (con la aguja y la jeringa acopladas) y el vial en el contenedor para objetos cortopunzantes. Si nodispone de un contenedor para desechar objetos cortopunzantes, puede utilizar un contenedor doméstico si este:
Desechar el contenedor lleno siguiendo las instrucciones de su médico o farmacéutico. Nota: Mantener siempre el contenedor para objetos cortopunzantes fuera de la vista y del alcance de los niños. |
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VYVGART 1.000 MG SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 1000 mgPrincipio activo: Efgartigimod alfaFabricante: Argenx B.V.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 20 mg/mlPrincipio activo: Efgartigimod alfaFabricante: Argenx B.V.Requiere recetaForma farmacéutica: COMPRIMIDO, 180 mgPrincipio activo: ácido micofenólicoFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para VYVGART 1.000 MG SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VYVGART 1.000 MG SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






 Contenido del envase
Contenido del envase