
VIVIDRIN 0,5 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS

Cómo usar VIVIDRIN 0,5 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Vividrin 0,5 mg/ml colirio en solución en envase unidosis
azelastina hidrocloruro
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico, farmacéutico o enfermero.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si necesita consejo o más información, consulte a su médico o farmacéutico.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Si después de 48 horas no se encuentra mejor o empeora, consulte a su médico.
Contenido del prospecto
- Qué es Vividrin y para qué se utiliza
- Qué necesita saber antes de empezar a usar Vividrin
- Cómo usar Vividrin
- Posibles efectos adversos
- Conservación de Vividrin
- Contenido del envase e información adicional
1. Qué es Vividrin y para qué se utiliza
Este medicamento contiene azelastina hidrocloruro, que pertenece a un grupo de conocidos como antihistamínicos. Los antihistamínicos previenen los efectos de sustancias como la histamina que son producidas por el organismo como parte de una reacción alérgica. La azelastina ha demostrado reducir la inflamación ocular.
Este medicamento se utiliza en las siguientes situaciones:
- Tratamiento y prevención de los trastornos oculares causados por la fiebre del heno (conjuntivitis alérgica estacional) en adultos y niños a partir de 4 años.
- Tratamiento de los trastornos oculares causados por la alergia a ácaros del polvo o pelo de animales (conjuntivitis alérgica perenne) en adultos y niños a partir de 12 años.
Este medicamento noes adecuado para el tratamiento de las infecciones oculares.
2. Qué necesita saber antes de empezar a usar Vividrin
No use Vividrin:
Si es alérgico a azelastina hidrocloruro o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar este medicamento:
- Si no está seguro de que sus síntomas oculares estén causados por una alergia. En particular, si solo afectan a un ojo, si su visión ha empeorado, o si tiene dolor ocular y no tiene síntomas nasales, posiblemente se trate de una infección en lugar de una alergia
- Si los síntomas empeoran o duran más de 48 horas sin mejoría notable a pesar de estar utilizando este medicamento
- Si utiliza lentes de contacto.
Niños y adolescentes
Este medicamento no se debe utilizar en niños menores de 4 años para el tratamiento y prevención de los trastornos oculares relacionados con la fiebre del heno (conjuntivitis alérgica estacional).
Este medicamento no se debe utilizar en niños menores de 12 años para el tratamiento de los trastornos oculares causados por la alergia a ácaros del polvo o pelo de animales (conjuntivitis alérgica perenne).
Otros medicamentos y Vividrin
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Se desconoce si este medicamento puede verse afectado por otros medicamentos.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
Puede notar visión borrosa durante un breve periodo de tiempo inmediatamente después de la aplicación de este medicamento. Si esto ocurre, espere hasta que vuelva a ver bien antes de conducir o utilizar maquinaria.
3. Cómo usar Vividrin
Siga exactamente las instrucciones de administración de este medicamento contenidas en este prospecto o indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico. Recuerde: este medicamento solo se debe aplicar en los ojos.
La dosis recomendada es:
Trastornos oculares causados por la fiebre del heno (conjuntivitis alérgica estacional)
Uso en adultos y niños a partir de los 4 años de edad.
La dosis habitual es una gota en cada ojo, por la mañana y por la noche.
Si prevé una exposición al polen, antes de salir al exterior puede administrarse la dosis habitual de este medicamento como medida preventiva.
Trastornos oculares causados por una alergia (conjuntivitis alérgica [perenne] no estacional)
Uso en adultos y niños a partir de los 12 años de edad.
La dosis habitual es una gota en cada ojo, por la mañana y por la noche.
Si sus síntomas son graves, su médico puede aumentar la dosis a una gota en cada ojo hasta cuatro veces al día.
Se debería notar alivio de los síntomas de la conjuntivitis alérgica al cabo de 15-30 minutos.
Método de administración (aplicación del colirio de este medicamento)
Para facilitar la correcta aplicación del colirio, las primeras veces puede ser útil sentarse delante de un espejo de forma que pueda ver lo que está haciendo.
- Lávese las manos.
- Limpie suavemente el contorno de sus ojos con un pañuelo para eliminar cualquier resto de humedad.
- Separe con cuidado un envase unidosis de la parte superior de la tira (Fig. 1) y ábralo. Gire y separe la parte superior de la unidad unidosis (sin tirar de ella) (Fig. 2).
- Incline la cabeza hacia atrás.
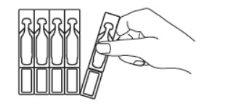
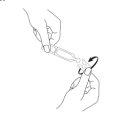
Fig. 1 Fig. 2
- Tire suavemente del párpado inferior hacia abajo.
- Coloque con cuidado una gota dentro de la zona media del párpado inferior. Procure que el envase no toque el ojo para evitar que se contamine (Fig. 3).
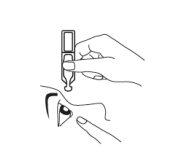
Fig. 3.
- Suelte el párpado inferior y presione suavemente el ángulo interno del ojo contra el puente de la nariz. Mientras el dedo presiona la nariz, parpadee lentamente unas cuantas veces para que la gota se extienda por toda la superficie del ojo.
- Seque el exceso de medicamento con un pañuelo.
- Repita el procedimiento con el otro ojo.
Duración del tratamiento
Si es posible, utilice este medicamento de manera regular hasta que los síntomas hayan desaparecido. Si interrumpe el uso de este medicamento es probable que sus síntomas reaparezcan.
No utilice este medicamento durante más de 6 semanas.
Si usa más Vividrin de lo que debe:
Si usted se administra demasiado Vividrin en el ojo, lo más probable es que no presente ningún problema. Si tiene cualquier duda, consulte a su médico. En caso de ingestión accidental de este medicamento, contacte inmediatamente con su médico o acuda al servicio de urgencias del hospital más próximo.
Si olvidó usar Vividrin
Use su colirio tan pronto como lo recuerde. Aplique la siguiente dosis en el horario habitual. No debe aplicarse una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento con Vividrin
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10personas):
Irritación leve (escozor, picor, lagrimeo) en los ojos después del uso de este medicamento. Estos efectos no deberían durar mucho tiempo.
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100personas):
Sabor amargo en la boca. Este efecto debería desaparecer rápidamente, en especial si toma un refresco.
Efectos adversos muy raros (pueden afectar hasta 1 de cada 10.000personas):
Reacción alérgica (como erupción y picor).
Comunicación de efectos adversos
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Vividrin
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja de cartón y en el envase, después de «CAD».
Utilice el envase unidosis una sola vez.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los
medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
No conservar a temperatura superior a 25 °C.
6. Contenido del envase e información adicional
Composición de Vividrin
- El principio activo es azelastina hidrocloruro.
Cada mililitro contiene 0,5 mg de azelastina hidrocloruro.
Cada gota contiene 0,018 mg de azelastina hidrocloruro.
- Los demás componentes son:
Solución de sorbitol al 70 % (no cristalizable), hipromelosa, edetato de disodio, hidróxido de sodio y agua para preparaciones inyectables.
Aspecto de Vividrin y contenido del envase
Este medicamento es una solución incolora y transparente que se presenta en un envase unidosis de LDPE transparente con un volumen de 0,6 ml.
Cada caja de cartón contiene 10, 20, 30 o 60 envases unidosis.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
BAUSCH + LOMB IRELAND LIMITED
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Irlanda
Representante Local en España
Bausch & Lomb S.A.
Avda. Valdelaparra, nº 4
28108 Alcobendas
Madrid. España.
Tel: 91 – 657 63 00
Responsable de la fabricación:
DR. GERHARD MANN. CHEM.
Brunsbütteler Damm 165/173,
13581 Berlin.
Alemania
Este medicamento está autorizado en los Estados miembros del Espacio Económico Europeo con los siguientes nombres:
DE:Vividrin Azelastin EDO 0,5 mg/ml Augentropfen, Lösung im Einzeldosisbehältnis |
NL:Azelergo 0,5 mg/ml oogdruppels, oplossing
AT:Vividrin Azelastin EDO 0,5 mg/ml Augentropfen, Lösung im Einzeldosisbehältnis IT:Monodrin occhi SI:Alezaxin 0,5 mg/ml kapljice za oko, raztopina v enoodmernem vsebniku |
BE:Azelergo 0,5 mg/ml collyre en solution en récipient unidose |
LU:Azelergo 0,5 mg/ml collyre en solution en récipient unidose
PT:Vivilin
ES:Vividrin 0,5 mg/ml colirio en solución en envase unidosis
EE:Vividrin
Este prospecto ha sido revisado por última vez enmayo 2021
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
- País de registro
- Precio medio en farmacia17.61 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VIVIDRIN 0,5 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSISForma farmacéutica: COLIRIO, 0,5 mg/mlPrincipio activo: azelastineFabricante: Cooper Consumer Health B.V.Requiere recetaForma farmacéutica: COLIRIO, 0,5 mg/mlPrincipio activo: azelastineFabricante: Qualix Pharma S.L.Requiere recetaForma farmacéutica: COLIRIO, 0,5 mg/mlPrincipio activo: azelastineFabricante: Mabo Farma S.A.Requiere receta
Médicos online para VIVIDRIN 0,5 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VIVIDRIN 0,5 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















