
VISUCOPT 20MG/ML + 5MG/ML COLIRIO EN SOLUCION

Cómo usar VISUCOPT 20MG/ML + 5MG/ML COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Visucopt 20mg/ml + 5mg/ml, colirio en solucióndorzolamida/timolol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Visucopt y para qué se utiliza
- Qué necesita saber antes de empezar a usar Visucopt
- Cómo usar Visucopt
- Posibles efectos adversos
- Conservación de Visucopt
- Contenido del envase e información adicional
1. Qué es Visucopt y para qué se utiliza
Visucopt contiene dos principios activos: dorzolamida y timolol
- La dorzolamida pertenece a un grupo de medicamentos denominados «inhibidores de la anhidrasa carbónica».
- El timolol pertenece a un grupo de medicamentos llamados «betabloqueantes».
Estos medicamentos disminuyen la presión ocular de diferentes maneras.
Visucopt se prescribe para reducir la presión ocular elevada en el tratamiento del glaucoma cuando el uso de un betabloqueante solo no es suficiente.
2. Qué necesita saber antes de empezar a usar Visucopt
No use Visucopt
- si es alérgico a los principios activos (clorhidrato de dorzolamida, maleato de timolol) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
- si tiene o ha tenido en el pasado problemas respiratorios como asma o bronquitis obstructiva crónica grave (una enfermedad pulmonar grave que puede causar sibilancias, dificultad para respirar o tos prolongada)
- si tiene una frecuencia cardiaca baja, insuficiencia cardiaca o trastornos del ritmo cardiaco (frecuencia cardiaca irregular)
- si tiene problemas renales graves o insuficiencia renal grave, o antecedentes de cálculos renales
- si tiene una acidez excesiva de la sangre, causada por la acumulación de cloruro (acidosis hiperclorémica)
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Visucopt si tiene o ha tenido en el pasado:
- enfermedades cardiovasculares/cardiopatía coronaria (los síntomas pueden consistir en dolor u opresión en el pecho, dificultad para respirar o asfixia), insuficiencia cardiaca, presión arterial baja; anomalías de la frecuencia cardiaca, como frecuencia cardiaca baja
- problemas respiratorios, asma o enfermedad pulmonar obstructiva crónica
- mala circulación sanguínea (como enfermedad de Raynaud o síndrome de Raynaud)
- diabetes, ya que el timolol, uno de los principios activos de Visucopt, puede enmascarar los signos y los síntomas de hipoglucemia
- hiperactividad de la glándula tiroides, ya que el timolol puede enmascarar los signos y los síntomas de este trastorno
Informe a su médico:
- si está usando Visucopt y tiene que someterse a una intervención quirúrgica, ya que el timolol puede alterar los efectos de algunos medicamentos utilizados durante la anestesia
- si le han diagnosticado miastenia grave (debilidad muscular)
- si presenta irritación ocular o problemas oculares nuevos como enrojecimiento del ojo o hinchazón de los párpados, póngase en contacto con su médico inmediatamente
- si presenta una infección ocular, sufre una lesión en el ojo, se somete a una intervención quirúrgica ocular o presenta otras reacciones, como la aparición de síntomas nuevos o el empeoramiento de síntomas preexistentes.
La instilación de Visucopt en el ojo puede afectar a todo el organismo.
Uso en niños y adolescentes
La experiencia con Visucopt en lactantes y niños es limitada.
Uso en personas de edad avanzada
En los estudios realizados con dorzolamida/timolol, los efectos de la combinación de los dos principios activos fueron similares en los pacientes de edad avanzada y en los más jóvenes.
Uso en pacientes con alteración de la función hepática (insuficiencia hepática)
Informe a su médico de cualquier problema hepático que tenga o haya tenido en el pasado.
Pruebas antidopaje
El uso de este medicamento puede producir resultados positivos en los controles de dopaje.
Otros medicamentos y Visucopt
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, incluso los adquiridos sin receta.
Visucopt puede afectar o verse afectado por otros medicamentos que esté utilizando, incluidos otros colirios para el tratamiento del glaucoma.
Informe a su médico si está utilizando o tiene previsto utilizar medicamentos para reducir la presión arterial, para tratar enfermedades cardiacas (cardiopatías) o para tratar la diabetes.
Informe a su médico:
- si está tomando medicamentos para reducir la presión arterial o para tratar enfermedades cardiacas (como antagonistas del calcio, betabloqueantes o digoxina).
- si está tomando medicamentos para tratar trastornos cardiacos o anomalías de la frecuencia cardiaca (como antagonistas del calcio, betabloqueantes o digoxina).
- si está utilizando otro colirio que contiene un principio activo perteneciente a la clase de los «betabloqueantes», pida más información a su médico.
- si está tomando otro medicamento para la hipertensión ocular (inhibidor de la anhidrasa carbónica, como acetazolamida).
- si está tomando medicamentos denominados pertenecientes a los «inhibidores de la monoaminooxidasa (IMAO)», utilizados para tratar la depresión.
- si está tomando un medicamento perteneciente a la clase de los «parasimpaticomiméticos» que le han recetado para ayudarle a orinar. Los parasimpaticomiméticos también son un tipo concreto de medicamento que a veces se utiliza para ayudar a normalizar la defecación.
- si está tomando «narcóticos» como morfina, utilizados para tratar dolores intensos.
- si está tomando medicamentos para tratar la diabetes.
- si está tomando antidepresivos conocidos, como fluoxetina y paroxetina.
- si está tomando «sulfamidas».
- si está tomando «quinidina», que se utiliza para tratar afecciones cardiacas y algunos tipos de paludismo.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Uso durante el embarazo
Visucopt no debe utilizarse durante el embarazo.
Uso durante la lactancia
No use Visucopt si está en periodo de lactancia. El timolol puede pasar a la leche materna.
Conducción y uso de máquinas
La influencia de Visucopt sobre la capacidad para conducir y utilizar máquinas es pequeña. Sin embargo, existen efectos adversos asociados al uso de Visucopt, como visión borrosa transitoria, que pueden afectar a su capacidad para conducir o utilizar máquinas. No conduzca ni utilice máquinas hasta que se encuentre bien o hasta que la visión se haya aclarado.
Visucopt contiene cloruro de benzalconio
.
Este medicamento contiene 0,075 mg de cloruro de benzalconio en cada mililitro, equivalente a 0,375 mg/5 ml. El cloruro de benzalconio se puede absorber por las lentes de contacto blandas alterando su color. Retirar las lentes de contacto antes de usar este medicamento y esperar 15 minutos antes de volver a colocarlas.
El cloruro de benzalconio puede causar irritación ocular, especialmente si padece de ojo seco u otras enfermedades de la córnea (capa transparente de la zona frontal del ojo). Consulte a su médico si siente una sensación extraña, escozor o dolor en el ojo después de usar este medicamento
3. Cómo usar Visucopt
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico. Su médico determinará la dosis y la duración adecuadas del tratamiento.
La dosis recomendada es de una gota de Visucopt en el ojo o los ojos afectados por la mañana y por la noche.
Si está utilizando Visucopt junto con otros colirios, deben instilarse con 10 minutos de diferencia como mínimo.
No cambie la dosis del medicamento sin consultar a su médico.
No deje que el tapón del frasco toque el ojo ni las zonas que lo rodean. Podría contaminarse con bacterias que pueden producir infecciones oculares que causan daños graves en el ojo, incluso pérdida de visión. Para evitar una posible contaminación, lávese las manos antes de utilizar este medicamento y mantenga el frasco alejado del contacto con cualquier superficie. Si cree que su medicación puede estar contaminada o si contrae una infección ocular, póngase en contacto con su médico inmediatamente para consultarle si debe seguir utilizando este frasco.
Instrucciones de uso
No utilice el recipiente si el anillo de plástico de seguridad que rodea el cuello del frasco está ausente o roto.
- Lávese las manos y siéntese o póngase de pie en una postura cómoda.
- Retire el tapón. Tenga especial cuidado de que la punta del cuentagotas no toque el ojo, la piel alrededor del ojo ni los dedos.
- Incline la cabeza hacia atrás, mantenga el envase boca abajo sobre el ojo y acerque la punta del frasco al ojo, sin tocarlo.
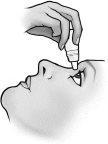
- Tire del párpado inferior hacia abajo y mire hacia arriba. Presione ligeramente el frasco para dispensar una sola gota en el ojo, de acuerdo con las instrucciones de su médico. Suelte el párpado inferior.
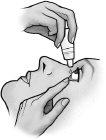
- Cierre el ojo y oprima con el dedo la esquina interna del ojo con su dedo, durante aproximadamente dos minutos. Esto ayuda a evitar que la gota llegue al resto del cuerpo.
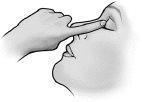
- Repita el proceso en el otro ojo si su médico se lo ha indicado.
- Cierre bien el tapón. No lo apriete en exceso para evitar dañar el frasco o el tapón.
- El dispensador de gotas está configurado para administrar una sola gota cada vez; por lo tanto, NO debe ampliar el orificio del dispensador de gotas.
Si usa más Visucopt del que debe
Si se pone demasiadas gotas en el ojo o se traga el contenido del envase, es posible que se maree, que tenga dificultad para respirar o que note una ralentización de la frecuencia cardiaca. Póngase en contacto con su médico inmediatamente.
En caso de sobredosis o ingestión accidental, acuda a un centro médico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad utilizada.
Si olvidó usar Visucopt
Es importante que use Visucopt según lo prescrito por su médico. Si se olvida de una dosis, adminístrela lo antes posible. Sin embargo, si ya es casi la hora de la siguiente dosis, sáltese la dosis olvidada y continúe con la pauta posológica habitual. No use una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento con Visucopt
Si desea dejar de usar este medicamento, hable primero con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
El timolol es absorbido por la sangre, que lo distribuye por todo el cuerpo, de forma similar a otros medicamentos oftálmicos. Esto puede causar efectos adversos similares a los observados con medicamentos del mismo grupo (betabloqueantes) tomados por vía oral. La incidencia de efectos adversos tras la administración ocular es menor que con la administración oral.
Si experimenta reacciones alérgicas, como hinchazón de la cara y las extremidades con dificultad para respirar o tragar, urticaria o erupción pruriginosa, erupción localizada y generalizada, picor o una reacción alérgica grave y repentina potencialmente mortal, deje de usar este medicamento y acuda al médico inmediatamente.
Se han notificado los siguientes efectos adversos con colirios de dorzolamida/timolol o con uno de sus componentes durante los ensayos clínicos o durante la experiencia posterior a la comercialización:
Muy frecuentes(pueden afectar a más de 1 de cada 10 pacientes)
-ardor y escozor ocular
-alteración del gusto
Frecuentes(pueden afectar hasta a 1 de cada 10 pacientes)
-cefalea
-enrojecimiento dentro y alrededor de los ojos, lagrimeo o picor en los ojos, erosión corneal (lesión de la capa situada en la parte anterior del globo ocular), hinchazón o irritación dentro y alrededor de los ojos, sensación de cuerpo extraño en el ojo, disminución de la sensibilidad corneal (no darse cuenta de que hay algo dentro del ojo y ausencia de dolor), dolor ocular, sequedad ocular, visión borrosa
-sinusitis (sensación de tensión o plenitud en la nariz)
-debilidad/cansancio y fatiga
Poco frecuentes(pueden afectar hasta a 1 de cada 100 pacientes)
-depresión
-mareo, desmayo
-inflamación del iris (una parte del ojo), alteraciones visuales (incluidos cambios refractivos en algunos casos debido a la retirada del tratamiento con mióticos)
-frecuencia cardiaca lenta
-dificultad para respirar (disnea)
-indigestión
-cálculos renales
Raros(pueden afectar hasta a 1 de cada 1.000 pacientes)
-reacciones de tipo alérgico como erupción cutánea, urticaria, picor, en casos raros hinchazón de labios, ojos y boca, sibilancias, o reacciones cutáneas graves (síndrome de Stevens Johnson, necrólisis epidérmica tóxica)
-problemas para dormir, pesadillas, pérdida de memoria
-isquemia cerebral (disminución del riego sanguíneo al cerebro), aumento de los signos y síntomas de miastenia grave (trastorno muscular), disminución del deseo sexual, hormigueo o entumecimiento de las manos o los pies
-miopía temporal que puede resolverse al interrumpir el tratamiento, desprendimiento de la capa situada por debajo de la retina y que contiene vasos sanguíneos después de la cirugía de filtración, lo que puede causar alteraciones visuales debido a acumulación de líquido, caída de los párpados, visión doble, formación de costras en los párpados, inflamación de la córnea (lo que provoca alteraciones visuales), presión ocular baja
-ruidos en el oído
-cambios en el ritmo o la velocidad de la frecuencia cardiaca, insuficiencia cardiaca congestiva (enfermedad del corazón, con dificultad para respirar e hinchazón de los pies y las piernas debido a la acumulación de líquido), edema (acumulación de líquido), dolor torácico, infarto de miocardio, tensión arterial baja, contracción excesiva de los vasos sanguíneos (fenómeno de Raynaud), hinchazón o frialdad de las manos y los pies, reducción de la circulación en los brazos y las piernas, calambres en las piernas o dolor en las piernas al caminar (claudicación), ictus
-dificultad para respirar, deterioro de la función pulmonar, moqueo o congestión nasal, hemorragia nasal, constricción de las vías respiratorias pulmonares, tos
-irritación de garganta, sequedad de boca, diarrea
-inflamación de la piel debida al contacto con determinadas sustancias (dermatitis de contacto), caída del cabello, erupción cutánea de color blanco plateado (erupción psoriasiforme)
-lupus eritematoso sistémico (una enfermedad inmunitaria que puede causar inflamación de órganos internos)
-Enfermedad de La Peyronie (que puede hacer que el pene se curve)
Otros efectos adversos enumerados son las reacciones habitualmente observadas con la clase farmacológica correspondiente a los betabloqueantes oftálmicos:
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
-niveles bajos de glucosa en sangre
-insuficiencia cardiaca, un tipo de trastorno del ritmo cardiaco
-dolor abdominal, vómitos
-dolor muscular no causado por el ejercicio
-disfunción sexual
-sibilancias
-sensación de cuerpo extraño ocular (sensación de tener algo dentro del ojo)
-latidos cardiacos fuertes, que pueden ser rápidos o irregulares (palpitaciones)
-aumento de la frecuencia cardiaca
-aumento de la tensión arterial
Comunicación de efectos adversos
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es.. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Visucopt
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar en el embalaje original para protegerlo de la luz.
No utilice este medicamento después de la fecha de caducidad (CAD) que aparece en la caja y en el frasco. La fecha de caducidad es el último día del mes que se indica.
Una vez abierto el frasco, no conserve el medicamento a más de 25 °C y utilícelo en un plazo de 28 días; transcurrido este periodo, deseche el medicamento que no haya usado. Anote la fecha de la primera apertura en el espacio correspondiente del envase.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda, pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Cada ml de solución contiene:
- 20 mg del principio activo dorzolamida 20 mg (correspondientes a 22,26 mg de clorhidrato de dorzolamida) y 5 mg del principio activo timolol (correspondientes a 6,83 mg de maleato de timolol).
- Los demás componentes son manitol, citrato de sodio, hidroxietilcelulosa, cloruro de benzalconio, hidróxido de sodio y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Visucopt es una solución transparente e incolora que se presenta en un frasco de polietileno.
Cada envase contiene un frasco de 5 ml.
Titular de la autorización de comercialización
VISUfarma S.p.A.
Via Alberto Cadlolo 21-00136 Roma,
Italia
Responsable de la fabricación
Genetic S.p.A.,
Contrada Canfora,
84084 Fisciano (SA), Italia
Fecha de la última revisión de este prospecto:Marzo 2023
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
…
- País de registro
- Precio medio en farmacia12.11 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VISUCOPT 20MG/ML + 5MG/ML COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 10 mg/ml + 5 mg/mlPrincipio activo: timolol, combinationsFabricante: Novartis Europharm LimitedRequiere recetaForma farmacéutica: COLIRIO, 0,3 mg/ml + 5 mg/mlPrincipio activo: timolol, combinationsFabricante: Brill Pharma S.L.Requiere recetaForma farmacéutica: COLIRIO, 0,3 mg/ml+5mg/mlPrincipio activo: timolol, combinationsFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para VISUCOPT 20MG/ML + 5MG/ML COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VISUCOPT 20MG/ML + 5MG/ML COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















