
VENLAFAXINA RETARD TEVA-RATIOPHARM 225 MG CAPSULAS DURAS DE LIBERACION PROLONGADA EFG

Cómo usar VENLAFAXINA RETARD TEVA-RATIOPHARM 225 MG CAPSULAS DURAS DE LIBERACION PROLONGADA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Venlafaxina Retard Teva-ratiopharm y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Venlafaxina Retard ratiopharm
- Cómo tomar Venlafaxina Retard ratiopharm
- Posibles efectos adversos
- Conservación de Venlafaxina Retard Teva-ratiopharm
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
Venlafaxina RetardTeva-ratiopharm225 mg cápsulas duras de liberación prolongada EFG
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Venlafaxina Retard Teva-ratiopharm y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Venlafaxina Retard Teva-ratiopharm
- Cómo tomar Venlafaxina Retard Teva-ratiopharm
- Posibles efectos adversos
- Conservación de Venlafaxina Retard Teva-ratiopharm
- Contenido del envase e información adicional
1. Qué es Venlafaxina Retard Teva-ratiopharm y para qué se utiliza
Venlafaxina Retard Teva-ratiopharm contiene el principio activo venlafaxina.
Venlafaxina Retard Teva-ratiopharm es un antidepresivo que pertenece a un grupo de medicamentos denominados inhibidores de la recaptación de serotonina y norepinefrina (ISRNs). Este grupo de medicamentos se utiliza para tratar la depresión y otros estados tales como los trastornos de ansiedad. Se cree que las personas que están deprimidas y/o padecen ansiedad tienen niveles inferiores de serotonina y norepinefrina en el cerebro. No se comprende completamente cómo funcionan los antidepresivos, pero pueden ayudar a aumentar los niveles de serotonina y norepinefrina en el cerebro.
Venlafaxina Retard Teva-ratiopharm es un tratamiento para adultos con depresión. También es un tratamiento para adultos con los siguientes trastornos de ansiedad: trastorno de ansiedad generalizada, trastorno de ansiedad social (miedo o evitación de las situaciones sociales), trastorno de pánico (ataques de pánico). Tratar los trastornos depresivos y de ansiedad adecuadamente es importante para ayudarle a sentirse mejor. Si no se trata, puede que su estado no desaparezca o puede agravarse y volverse más difícil de tratar.
2. Qué necesita saber antes de empezar a tomar Venlafaxina Retard ratiopharm
No tome Venlafaxina Retard ratiopharm:
- Si es alérgico a venlafaxina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
- Si también está tomando, o ha tomado en los últimos 14 días, algún medicamento conocido como inhibidor de la monoaminooxidasa (IMAO) irreversible, usado para tratar la depresión o la enfermedad de Parkinson. Tomar un IMAO irreversible junto con Venlafaxina Retard ratiopharm, puede producir efectos adversos graves o incluso potencialmente mortales. Además, debeesperar al menos 7 días una vez que deje de tomar Venlafaxina Retard ratiopharm antes de tomar cualquier IMAO (ver también la sección “Toma de Venlafaxina Retard ratiopharm con otros medicamentos” y la información en esa sección sobre “Síndrome Serotoninérgico”).
Advertencias y precauciones
Consulte a su médico o farmacéutico antesde empezar a tomar Venlafaxina Retard ratiopharm:
- Si utiliza otros medicamentos que tomados junto con Venlafaxina Retard ratiopharm podrían aumentar el riesgo de desarrollar síndrome serotoninérgico (ver la sección “Toma de Venlafaxina Retard ratiopharm con otros medicamentos”)
- Si tiene problemas en los ojos, tales como ciertos tipos de glaucoma (aumento de la presión en el ojo)
- Si tiene antecedentes de tensión arterial alta
- Si tiene antecedentes de problemas de corazón
- Si ha sido informado de que el ritmo de su corazón está alterado
- Si tiene antecedentes de ataques (convulsiones)
- Si tiene antecedentes de niveles bajos de sodio en sangre (hiponatremia)
- Si tiene tendencia a desarrollar cardenales o tendencia a sangrar fácilmente (antecedentes de trastornos hemorrágicos), o si está embarazada (ver “Embarazo y lactancia”) o si está usando otros medicamentos que pueden aumentar el riesgo de hemorragia p.ej., warfarina (usado para prevenir coágulos de sangre)
- Si tiene antecedentes de, o si alguien de su familia ha tenido, manía o trastorno bipolar (sentirse sobreexcitado o eufórico)
- Si tiene antecedentes de comportamiento agresivo.
Venlafaxina Retard ratiopharm puede provocar una sensación de inquietud o una dificultad para sentarse o estar quieto durante las primeras semanas de tratamiento. Debe consultar a su médico si le ocurre esto.
No beba alcohol durante el tratamiento con venlafaxina, ya que puede provocar cansancio extremo e inconsciencia. La toma junto con ciertos medicamentos y/o con alcohol puede empeorar los síntomas de la depresión y de otras afecciones, como los trastornos de ansiedad.
Pensamientos de suicidio y empeoramiento de su depresión o trastorno de ansiedad
Si está deprimido y/o tiene trastornos de ansiedad, a veces puede tener pensamientos de hacerse daño o suicidarse. Estos pensamientos pueden aumentar al comenzar a tomar antidepresivos, debido a que todos estos medicamentos tardan un tiempo en hacer efecto, normalmente unas dos semanas, pero a veces puede ser más tiempo. Estos pensamientos también pueden producirse cuando su dosis disminuye o durante la interrupción del tratamiento con venlafaxina.
Es más probable que le suceda esto:
- Si ha tenido previamente pensamientos de suicidio o de hacerse daño
- Si es un adulto joven. La información de los ensayos clínicos ha mostrado un aumento del riesgo de conductas suicidas en adultos jóvenes (menos de 25 años de edad) con enfermedades psiquiátricas que se trataron con antidepresivos.
Si tiene pensamientos de hacerse daño o suicidarse en cualquier momento, póngase en contacto con su médico o acuda a un hospital directamente.
Puede encontrar útil contarle a un familiar o amigo cercano que está deprimido o que tiene un trastorno de ansiedad, y pedirles que lean este prospecto. Puede pedirles también que le digan si piensan que su depresión o ansiedad está empeorando, o si están preocupados acerca de los cambios en su conducta.
Sequedad bucal
Se ha informado de sequedad de boca en el 10% de pacientes tratados con venlafaxina. Esto puede aumentar el riesgo de que se pudran los dientes (caries). Por tanto, debe tener cuidado con su higiene dental.
Diabetes
Sus niveles de glucosa en sangre pueden ser alterados por Venlafaxina Retard ratiopharm. Por lo tanto, las dosis de sus medicamentos para la diabetes pueden necesitar ser ajustadas.
Problemas sexuales
Algunos medicamentos del grupo al que pertenece Venlafaxina Retard ratiopharm (llamados IRSN) pueden causar síntomas de disfunción sexual (ver sección 4). En algunos casos, estos síntomas persisten después de suspender el tratamiento.
Niños y adolescentes
Venlafaxina Retard ratiopharm no deberá utilizarse normalmente en el tratamiento de niños y adolescentes menores de 18 años. Además, debe saber que en pacientes menores de 18 años existe un mayor riesgo de efectos adversos como intentos de suicidio, ideas de suicidio y hostilidad (predominantemente agresión, comportamiento de confrontación e irritación) cuando ingieren esta clase de medicamentos. Pese a esto, su médico puede prescribir este medicamento a pacientes menores de 18 años cuando decida que es lo más conveniente para el paciente. Si su médico ha prescrito este medicamento a un paciente menor de 18 años, y desea discutir esta decisión, por favor, vuelva a su médico. Debe informar a su médico si se desarrollan o empeoran alguno de los síntomas enumerados anteriormente cuando estos pacientes menores de 18 años están tomando Venlafaxina Retard ratiopharm. Además, la seguridad a largo plazo en relación con el crecimiento, la madurez y el desarrollo cognitivo y conductual, no ha sido demostrada en este grupo de edad.
Toma de Venlafaxina Retard ratiopharm con otros medicamentos
Informe a su médico o farmacéutico si está tomando/usando, ha tomado/usado recientemente o pudiera tener que tomar/usar cualquier otro medicamento.
Su médico debe decidir si puede tomar Venlafaxina Retard ratiopharm con otros medicamentos.
No comience ni deje de tomar cualquier medicamento, incluyendo los que se venden sin receta, remedios naturales y a base de plantas, antes de comprobarlo con su médico o farmacéutico.
- Inhibidores de la monoaminooxidasa usados para tratar la depresión o la enfermedad de Parkinson no se deben tomar con Venlafaxina Retard ratiopharm. Dígale a su médico si ha tomado alguno de estos medicamentos en los últimos 14 días. (IMAO: ver sección “Qué necesita saber antes de empezar a tomar Venlafaxina Retard ratiopharm”).
- Síndrome serotoninérgico:
- Un estado potencialmente mortal, o reacciones parecidas al Síndrome Neuroléptico Maligno (SNM) (ver sección “Posibles efectos adversos”) pueden producirse con el tratamiento con venlafaxina, particularmente cuando se toma con otros medicamentos.
Ejemplos de estos medicamentos incluyen:
- Triptanes (usados para la migraña)
- Otros medicamentos para tratar la depresión, por ejemplo, ISRN, ISRS, antidepresivos tricíclicos o medicamentos que contienen litio
- Medicamentos que contienen anfetaminas (usadas para tratar el trastorno por déficit de atención con hiperactividad (TDAH), la narcolepsia y la obesidad)
- Medicamentos que contienen linezolid, un antibiótico (usado para tratar infecciones)
- Medicamentos que contienen moclobemida, un IMAO (usado para tratar la depresión)
- Medicamentos que contienen sibutramina (usado para la pérdida de peso)
- Medicamentos que contienen buprenorfina, tramadol, fentanilo, tapentadol, petidina o pentazocina (usados para tratar el dolor fuerte)
- Medicamentos que contienen dextrometorfano (usado para tratar la tos)
- Medicamentos que contienen metadona (usado para el tratamiento de la adicción a opiáceos o para el tratamiento del dolor fuerte)
- Medicamentos que contienen azul de metileno (usado para tratar los niveles elevados de metahemoglobina en sangre)
- Productos que contienen hierba de San Juan (también denominada “Hypericum perforatum”, un remedio natural o a base de plantas usado para tratar la depresión leve)
- Productos que contienen triptófano (usados para problemas tales como sueño y depresión)
- Antipsicóticos (usados para tratar una enfermedad con síntomas tales como oír, ver o sentir cosas que no existen, creencias erróneas, suspicacia inusual, razonamiento poco claro ytendencia al retraimiento) y otros antagonistas de la dopamina como metoclopramida (usado para tratar náuseas y vómitos).
Los signos y síntomas del síndrome serotoninérgico pueden incluir una combinación de los siguientes:
inquietud, alucinaciones, pérdida de coordinación, latido cardiaco rápido, aumento de la temperatura corporal, cambios rápidos en la tensión arterial, reflejos hiperactivos, diarrea, coma, náuseas, vómitos.
En su forma más grave, el síndrome serotoninérgico puede parecerse al síndrome neuroléptico maligno (SNM). Los signos y síntomas del SNM pueden incluir una combinación de fiebre, latido cardiaco rápido, sudoración, rigidez grave de los músculos, confusión, aumento de las enzimas de los músculos (determinado por un análisis de sangre).
Informe a su médico inmediatamente, o acuda a urgencias del hospital más cercano si cree que está experimentando el síndrome serotoninérgico.
Debe informar a su médico si está tomando medicamentos que puedan alterar el ritmo de su corazón.
Algunos ejemplos de estos medicamentos incluyen:
- Antiarrítmicos como quinidina, amiodarona, sotalol o dofetilida (utilizados para tratar el ritmo del corazón alterado)
- Antipsicóticos como tioridazina (ver también síndrome serotoninérgico más arriba)
- Antibióticos como eritromicina o moxifloxacino (utilizados para tratar infecciones por bacterias)
- Antihistamínicos (utilizados para tratar la alergia).
Los siguientes medicamentos también pueden interaccionar con Venlafaxina Retard ratiopharm y deben usarse con precaución. Es especialmente importante mencionar a su médico o farmacéutico si está tomando medicamentos que contienen:
- Ketoconazol (un medicamento antifúngico)
- Haloperidol o risperidona (para tratar estados psiquiátricos)
- Metoprolol (un betabloqueante para tratar la tensión arterial elevada y problemas cardiacos).
Informe a su médico si está tomando anticonceptivos orales.
Toma de Venlafaxina Retard ratiopharm con alimentos, bebidas y alcohol
Venlafaxina Retard ratiopharm debe tomarse con alimentos (ver sección 3 “Cómo tomar Venlafaxina Retard ratiopharm”).
No beba alcohol durante el tratamiento con venlafaxina. La toma junto con alcohol puede provocar cansancio extremo e inconsciencia, y puede empeorar los síntomas de la depresión y de otras afecciones, como los trastornos de ansiedad.
Además, el alcohol puede afectar al recubrimiento del comprimido, lo que puede dar lugar a una liberacaión rápida de una cantidad relativamente grande de venlafaxina, pudiendo provocar toxicidad o sobredosis (ver “Si toma más Venlafaxina Retard ratiopharm del que debe”) e impidiendo que la cápsula tenga efecto hasta la próxima toma prevista.
Embarazo y lactancia
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
Sólo debe tomar Venlafaxina Retard ratiopharm tras discutir con su médico los posibles beneficios y los posibles riesgos para el niño no nacido.
Asegúrese de que su comadrona y/o médico sabe que está tomando Venlafaxina Retard ratiopharm. Cuando se toman durante el embarazo, fármacos similares (ISRS) pueden aumentar el riesgo de un estado grave en bebés, denominado hipertensión pulmonar persistente del recién nacido (HPPRN), haciendo que el bebé respire más rápido y se ponga morado. Estos síntomas normalmente comienzan durante las primeras 24 horas tras el nacimiento del bebé. Si esto le ocurre a su bebé debe ponerse inmediatamente en contacto con su comadrona y/o médico.
Si está tomando este medicamento durante el embarazo, además de problemas con la respiración, otro síntoma que su bebé podría tener cuando nazca es problema en la alimentación. Si su bebé tiene estos síntomas cuando nazca y está preocupada, póngase en contacto con su médico y/o comadrona quienes podrán aconsejarla.
Si toma Venlafaxina Retard ratiopharm en la etapa final del embarazo puede producirse un mayor riesgo de sangrado vaginal abundante poco después del parto, especialmente si tiene antecedentes de alteraciones hemorrágicas. Su médico o matrona deben saber que usted está tomando Venlafaxina Retard ratiopharm para poderle aconsejar.
Lactancia
Venlafaxina Retard ratiopharm pasa a la leche materna. Existe un riesgo de un efecto para el bebé. Por tanto, debe tratar el caso con su médico y él decidirá si debe interrumpir la lactancia o interrumpir el tratamiento con este medicamento.
Conducción y uso de máquinas
No conduzca ni maneje herramientas o máquinas hasta que sepa cómo le afecta este medicamento.
Venlafaxina Retard ratiopharm 225 mg contiene Carmoisina (E 122)
Puede causar recciones alérgicas.
3. Cómo tomar Venlafaxina Retard ratiopharm
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis inicial normal recomendada para el tratamiento de la depresión, trastorno de ansiedad generalizada y trastorno de ansiedad social es de 75 mg por día. Su médico puede aumentar gradualmente la dosis y si es necesario todavía más hasta un máximo de 375 mg al día para la depresión. Si se le está tratando por trastorno de pánico, su médico comenzará con una dosis inferior (37,5 mg) y después aumentará la dosis gradualmente. La dosis máxima para el trastorno de ansiedad generalizada, trastorno de ansiedad social y trastorno de pánico es de 225 mg/día.
Tome Venlafaxina Retard ratiopharm aproximadamente a la misma hora cada día, ya sea por la mañana o por la noche. Las cápsulas deben tragarse enteras con líquido y no deben abrirse, aplastarse, masticarse ni disolverse.
Venlafaxina Retard ratiopharm debe tomarse con comida.
Si tiene problemas de hígado o de riñón, hable con su médico ya que puede ser necesario que su dosis de este medicamento sea diferente.
No deje de tomar este medicamento sin consultarlo con su médico (ver sección “Si interrumpe el tratamiento con Venlafaxina Retard ratiopharm”).
Si toma más Venlafaxina Retard ratiopharm del que debe
En caso de sobredosis o ingestión accidental, contacte inmediatamente con su médico, farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20 indicando el medicamento y la cantidad ingerida.
La sobredosis puede poner en peligro su vida, especialmente con la toma simultánea de ciertos medicamentos y/o de alcohol (ver “Toma de Venlafaxina Retard ratiopharm con otros medicamentos”).
Los síntomas de una posible sobredosis pueden incluir palpitaciones, cambios en el nivel de vigilancia (que va desde somnolencia a coma), visión borrosa, convulsiones o ataques y vómitos.
Si olvidó tomar Venlafaxina Retard ratiopharm
Si no se ha tomado una dosis, tómela en cuanto lo recuerde. Sin embargo, si ya es la hora de su siguiente dosis, sáltese la dosis perdida y tome sólo una única dosis como habitualmente. No tome una dosis doble para compensar las dosis olvidadas. No tome más de la cantidad diaria de Venlafaxina Retard ratiopharm, que le han recetado en un día.
Si interrumpe el tratamiento con Venlafaxina Retard ratiopharm
No deje de tomar su tratamiento ni reduzca la dosis sin el consejo de su médico, aun cuando se sienta mejor. Si su médico cree que ya no necesita Venlafaxina Retard ratiopharm, puede pedirle que reduzca la dosis lentamente antes de interrumpir el tratamiento totalmente. Se sabe que se producen efectos adversos cuando las personas dejan de utilizar este medicamento, especialmente cuando se deja de tomar repentinamente o si la dosis se reduce muy rápidamente. Algunos pacientes pueden experimentar síntomas como pensamientos suicidas, agresividad, cansancio, mareos, falta de estabilidad, dolor de cabeza, insomnio, pesadillas, sequedad de boca, pérdida de apetito, náuseas, diarrea, nerviosismo, agitación, confusión, zumbidos en los oídos, hormigueo o, en escasas ocasiones, sensaciones de descarga eléctrica, debilidad, sudoración, convulsiones o síntomas similares a la gripe, problemas con la vista y aumento de la tensión arterial (que puede provocar dolor de cabeza, mareos, zumbidos en los oídos, sudoración, etc.).
Su médico le aconsejará cómo debe interrumpir gradualmente el tratamiento con Venlafaxina Retard ratiopharm. Esto puede llevar varias semanas o meses. En algunos pacientes, la interrupción puede que tenga que producirse muy gradualmente durante meses o más. Si experimenta cualquiera de éstos u otros síntomas que le resulten molestos, consulte a su médico para que le aconseje.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si se produce cualquiera de los efectos siguientesdeje de tomar Venlafaxina Retard Teva-ratiopharm.Comuníqueselo a su médico inmediatamente, o acuda a urgencias del hospital más cercano:
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
- Hinchazón de la cara, boca, lengua, garganta, manos o pies y/o erupción hinchada con picor, dificultad al tragar o al respirar.
Raros (pueden afectar hasta 1 de cada 1.000 personas)
- Opresión en el pecho, ruido al respirar, dificultad al tragar o al respirar
- Erupción grave en la piel, picazón o urticaria (zonas elevadas de piel enrojecida o pálida que a menudo pica)
- Signos y síntomas del síndrome serotoninérgico que puede incluir agitación, alucinaciones, pérdida de coordinación, latidos rápidos del corazón, aumento de la temperatura corporal, cambios rápidos en la presión arterial, reflejos hiperactivos, diarrea, coma, náuseas, vómitos.
En su forma más grave, el síndrome serotoninérgico puede parecerse al Síndrome Neuroléptico Maligno (SNM). Los signos y síntomas del SNM pueden incluir una combinación de fiebre, latido rápido del corazón, sudoración, rigidez grave de los músculos, confusión, aumento de las enzimas de los músculos (determinado por un análisis de sangre)
- Signos de infección, como aumento de la temperatura, escalofríos, tiritona, cefalea, sudoración o síntomas similares a la gripe. Esto puede deberse a un trastorno de la sangre que puede derivar a un aumento del riesgo de infección
- Erupción grave que puede derivar en la formación de ampollas graves y a la descamación de la piel
- Dolor de músculos inexplicable, molestias o debilidad. Esto puede ser un signo de rabdomiolisis.
Frecuencia no conocida (no se puede estimar con los datos disponibles)
Signos y síntomas de un estado denominado “miocardiopatía por estrés” que puede incluir dolor en el pecho, dificultad para respirar, mareos, desmayos, latidos cardiacos irregulares.
Otros efectos adversos sobre los que usted debe informar a su médicoson (la frecuencia de estos efectos adversos están incluidos en la lista inferior “Otros efectos adversos que pueden ocurrir”):
- Tos, ruido al respirar y dificultad para respirar que pueden estar acompañados de un aumento de la temperatura
- Heces (deposiciones) alquitranosas o sangre en heces
- Picor, ojos o piel amarilla, orina oscura, que son síntomas de una inflamación del hígado (hepatitis)
- Problemas de corazón, como frecuencia cardiaca rápida o irregular, aumento de la presión arterial
- Problemas en los ojos, como visión borrosa, pupilas dilatadas
- Problemas en los nervios, como mareos, hormigueo, trastorno del movimiento (espasmos de los músculos y rigidez), convulsiones o ataques
- Problemas psiquiátricos, como hiperactividad y sensación inusual de sobreexcitación
- Efectos de retirada (ver sección “Cómo tomar Venlafaxina Retard Teva-ratiopharm, si interrumpe el tratamiento con Venlafaxina Retard Teva-ratiopharm”)
- Sangrado prolongado, si se corta o se hace una herida, puede tardar un poco más de lo normal en que pare el sangrado.
Otros efectos adversos que pueden ocurrir
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Mareo, dolor de cabeza, somnolencia
- Insomnio
- Náuseas, boca seca, estreñimiento
- Sudoración excesiva (incluyendo sudores nocturnos).
Frecuentes (pueden afectar hasta 1 de cada 10 personas)
- Disminución del apetito
- Confusión, sentirse extraño, falta de orgasmo, disminución de la libido, agitación, nerviosismo, sueños anómalos
- Temblor, una sensación de inquietud o incapacidad para permanecer sentado o estar quieto, hormigueo, percepción alterada del gusto, aumento del tono de los músculos
- Alteraciones visuales incluyendo visión borrosa, pupilas dilatadas, incapacidad del ojo para cambiar automáticamente el enfoque de objetos distantes a cercanos
- Zumbidos en los oídos (tinnitus)
- Latido cardiaco rápido, palpitaciones
- Aumento de la presión arterial, sofocos
- Dificultad para respirar, bostezos
- Vómitos, diarrea
- Erupción cutánea leve, picazón
- Aumento de la frecuencia urinaria, incapacidad para orinar, dificultades para orinar
- Irregularidades menstruales, tales como aumento del sangrado o aumento del sangrado irregular, eyaculación/orgasmo anómalos (varones), disfunción eréctil (impotencia)
- Debilidad (astenia), fatiga, escalofríos
- Aumento o pérdida de peso
- Aumento del colesterol.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
- Hiperactividad, pensamientos acelerados y disminución de la necesidad de dormir (manía).
- Alucinaciones, sentirse separado de la realidad, orgasmo anómalo, falta de sentimientos o emociones, sentirse sobreexcitado, rechinar de los dientes
- Desmayo, movimientos involuntarios de los músculos, alteración de la coordinación y del equilibrio
- Sentirse mareado (sobre todo al levantarse demasiado deprisa), disminución de la presión arterial
- Vómito de sangre, heces alquitranosas (deposiciones) o sangre en las heces, que puede ser un signo de hemorragia interna
- Sensibilidad a la luz del sol, cardenales, caída del cabello anómala
- Incapacidad para controlar la orina
- Rigidez, espasmos y movimientos involuntarios de los músculos
- Cambios ligeros en los niveles sanguíneos de enzimas del hígado
Raros (pueden afectar hasta 1 de cada 1.000 personas)
- Convulsiones o ataques
- Tos, ruido al respirar y falta de aliento que pueden estar acompañados de una temperatura alta.
- Desorientación y confusión a menudo acompañada de alucinaciones (delirium)
- Exceso de ingesta de agua (conocido como SIADH)
- Disminución de los niveles de sodio en sangre
- Dolor intenso en el ojo y visión reducida o borrosa
- Latido del corazón anómalo, rápido o irregular, que puede conducir a desmayos.
- Dolor grave de abdomen o de espalda (que puede indicar un problema grave en el intestino, hígado o páncreas).
- Picor, ojos o piel amarillentos, orina oscura, síntomas similares a la gripe, que son síntomas de inflamación del hígado (hepatitis).
Muy raros (pueden afectar hasta 1 de cada 10.000 personas)
- Sangrado prolongado, que puede deberse a un número reducido de plaquetas en la sangre que conduce a un aumento del riesgo de cardenales o hemorragias
- Producción anómala de leche materna.
- Sangrado inesperado, por ejemplo, sangrado de las encías, sangre en orina o en el vómito, o la aparición de cardenales o rotura de vasos sanguíneos (venas rotas)
Frecuencia no conocida (no se puede estimar con los datos disponibles)
- Ideación suicida y comportamiento suicida, se han notificado casos de ideación y comportamiento suicida durante el tratamiento con venlafaxina o poco después de la interrupción del tratamiento (ver sección 2. “Qué necesita saber antes de tomar Venlafaxina Retard Teva-ratiopharm”).
- Agresión.
- Vértigo
- Sangrado vaginal abundante poco después del parto (hemorragia posparto), ver “Embarazo y lactancia” en la sección 2 para más información.
Venlafaxina Retard Teva-ratiopharm produce algunas veces efectos no deseados de los que puede que no sea consciente, tales como aumentos de la presión arterial o un latido del corazón anómalo; cambios ligeros en los niveles sanguíneos de enzimas del hígado, sodio o colesterol. Con menos frecuencia, Venlafaxina Retard Teva-ratiopharm puede reducir la función de las plaquetas de la sangre, lo que conduce a un aumento del riesgo de aparición de cardenales o hemorragia. Por tanto, su médico puede desear realizar análisis de sangre ocasionalmente, en particular si ha estado tomando Venlafaxina Retard Teva-ratiopharm durante mucho tiempo.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: http://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Venlafaxina Retard Teva-ratiopharm
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Venlafaxina Retard ratiopharm 225 mg
- El principio activo es venlafaxina.
Cada cápsula de liberación prolongada contiene 225 mg de venlafaxina, como venlafaxina hidrocloruro.
- Los demás componentes son:
Contenido de la cápsula: celulosa microcristalina, povidona (K-90), talco, dióxido de silicio coloidal, estearato magnésico, etilcelulosa, copovidona.
Cubierta de la cápsula: gelatina, carmoisina (E 122), dióxido de titanio (E171), agua.
Tinta de impresión de la cápsula: shellac, propilenglicol, solución fuerte de amonio, laca de aluminio índigo carmín (E 132).
Aspecto del producto y contenido del envase
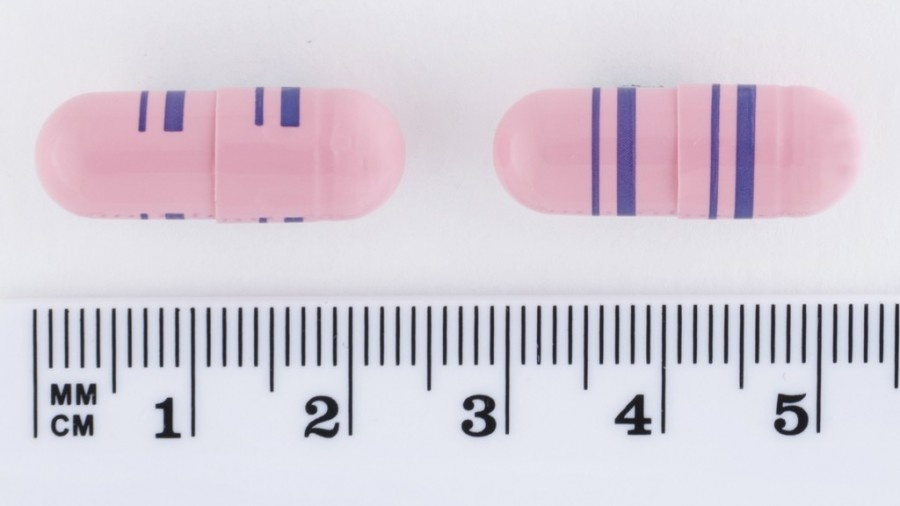
Venlafaxina Retard ratiopharm 225 mg son cápsulas de gelatina duras, de color rosa, opacas, con bandas azules circulares radiales, gruesas y finas en el cuerpo y en la tapa, conteniendo mini comprimidos.
Venlafaxina Retard ratiopharm 225 mg está disponible en:
Envases con blísteres de aluminio y aluminio formado en frío (OPA/Alu/PVC) conteniendo 28, 30, 30x1 cápsulas duras de liberación prolongada.
Envases con blísteres de PVC/PVdC blanco opaco y aluminio, conteniendo 28, 30, 30x1 cápsulas duras de liberación prolongada.
Envases con blísteres de PVC/Aclar blanco opaco y aluminio, conteniendo 28, 30, 30x1 cápsulas duras de liberación prolongada.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular
Teva Pharma, S.L.U.
C/Anabel Segura, 11. Edificio Albatros B, 1ª planta.
Alcobendas, 28108 - Madrid
Responsable de la fabricación
Merckle GmbH
Ludwig-Merckle-Straße 3,
89143 Blaubeuren
Alemania
Fecha de la última revisión de este prospecto: Mayo 2023
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http: //www.aemps.gob.es/
Puede acceder a información detallada y actualizada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el cartonaje. También puede acceder a esta información en la siguiente dirección de internet: https://cima.aemps.es/cima/dochtml/p/81364/P_81364.html
Código QR + URL
- País de registro
- Precio medio en farmacia28.74 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VENLAFAXINA RETARD TEVA-RATIOPHARM 225 MG CAPSULAS DURAS DE LIBERACION PROLONGADA EFGForma farmacéutica: CAPSULA LIBERACION MODIFICADA, 150 mg venlafaxina hidrocloruroPrincipio activo: venlafaxinaFabricante: Adamed Laboratorios S.L.U.Requiere recetaForma farmacéutica: CAPSULA LIBERACION MODIFICADA, 75 mg venlafaxina hidrocloruroPrincipio activo: venlafaxinaFabricante: Adamed Laboratorios S.L.U.Requiere recetaForma farmacéutica: COMPRIMIDO, 37,5 mg venlafaxinaPrincipio activo: venlafaxinaFabricante: Almirall S.A.Requiere receta
Médicos online para VENLAFAXINA RETARD TEVA-RATIOPHARM 225 MG CAPSULAS DURAS DE LIBERACION PROLONGADA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VENLAFAXINA RETARD TEVA-RATIOPHARM 225 MG CAPSULAS DURAS DE LIBERACION PROLONGADA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











