
TREMFYA 100 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar TREMFYA 100 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el usuario
Tremfya 100 mg solución inyectable en pluma precargada
guselkumab
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque
contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Tremfya y para qué se utiliza
- Qué necesita saber antes de empezar a usar Tremfya
- Cómo usar Tremfya
- Posibles efectos adversos
- Conservación de Tremfya
- Contenido del envase e información adicional
1. Qué es Tremfya y para qué se utiliza
Tremfya contiene el principio activo guselkumab, que es un tipo de proteína denominada anticuerpo monoclonal.
Este medicamento actúa bloqueando la actividad de una proteína llamada IL-23, que está presente en una cantidad mayor en las personas con psoriasis y artritis psoriásica.
Psoriasis en placas
Tremfya se usa para tratar a adultos con “psoriasis en placas” de moderada a grave, un trastorno inflamatorio que afecta a la piel y las uñas.
Tremfya puede mejorar los trastornos de la piel y la apariencia de las uñas y reduce los síntomas, tales como descamación, desprendimiento, exfoliación, picor, dolor y escozor.
Artritis psoriásica
Tremfya se utiliza para tratar una enfermedad llamada “artritis psoriásica”, una enfermedad inflamatoria de las articulaciones, a menudo acompañada de psoriasis. Si padece artritis psoriásica, le administrarán primero otros medicamentos. Si no responde lo suficientemente bien a estos medicamentos, le administrarán Tremfya para reducir los signos y síntomas de su enfermedad. Tremfya se puede utilizar solo o con otro medicamento llamado metotrexato.
El uso de Tremfya en la artritis psoriásica le beneficiará reduciendo los signos y síntomas de la enfermedad, ralentizando el daño del cartílago y el hueso de las articulaciones y mejorando su capacidad para realizar las actividades diarias normales.
2. Qué necesita saber antes de empezar a usar Tremfya
No use Tremfya
- Si es alérgico a guselkumab o a alguno de los demás componentes de este medicamento
(incluidos en la sección 6). Si piensa que puede ser alérgico, consulte a su médico antes de usar Tremfya
- Si tiene una infección activa, incluyendo tuberculosis activa
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de usar Tremfya:
- si está recibiendo tratamiento para una infección
- si tiene una infección persistente o que ha reaparecido
- si padece tuberculosis o ha mantenido un contacto cercano con alguien con tuberculosis
- si piensa que tiene una infección o síntomas de una infección (vea a continuación “Vigilancia de infecciones y reacciones alérgicas”)
- si se ha vacunado recientemente o tiene que ponerse una vacuna durante el tratamiento con Tremfya.
Si no está seguro de si alguna de las condiciones anteriores son aplicables en su caso, hable con su médico, farmacéutico o enfermero antes de utilizar Tremfya.
Siguiendo las indicaciones de su médico, antes de recibir Tremfya y cuando lo vaya a utilizar, puede necesitar hacerse análisis de sangre para comprobar si tiene elevados los niveles de las enzimas del hígado. Los aumentos de las enzimas del hígado pueden ser más frecuentes en los pacientes que reciben Tremfya cada 4 semanas que en los que reciben Tremfya cada 8 semanas (ver “Cómo usar Tremfya” en la sección 3).
Vigilancia de infecciones y reacciones alérgicas
Tremfya tiene potencial para producir reacciones adversas graves, incluyendo reacciones alérgicas e infecciones. Debe vigilar la aparición de signos de estos trastornos mientras esté recibiendo Tremfya.
Deje de usar Tremfya y consulte a su médico o busque ayuda médica inmediatamente si observa
cualquier signo que indique una posible reacción alérgica grave o una infección.
Los signos de infección pueden incluir fiebre o síntomas pseudogripales; dolores musculares; tos; dificultad para respirar; escozor al orinar u orinar con más frecuencia de la habitual; sangre en las flemas (moco); pérdida de peso; diarrea o dolor de estómago; piel caliente, enrojecida o dolorosa, o llagas en el cuerpo que son diferentes de las lesiones de psoriasis.
Se han producido reacciones alérgicas graves con Tremfya, que pueden incluir los siguientes síntomas, hinchazón de la cara, labios, boca, lengua o garganta, dificultad para tragar o respirar y habones (ver “Efectos adversos graves” en la sección 4).
Niños y adolescentes
No se recomienda la administración de Tremfya a niños y adolescentes menores de 18 años porque no hay estudios en este grupo de edad.
Otros medicamentos y Tremfya
Hable con su médico o farmacéutico:
- si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro
medicamento.
- si se ha vacunado recientemente o tiene que vacunarse. No debe recibir determinados tipos de vacunas (de organismos vivos) mientras use Tremfya.
Embarazo y lactancia
- No se debe usar Tremfya durante el embarazo ya que no se conocen los efectos de este medicamento en las mujeres embarazadas. Si es usted una mujer en edad fértil, se le recomienda que evite quedarse embarazada y que utilice un método de anticoncepción adecuado durante el tratamiento con Tremfya y al menos las 12 semanas siguientes a la última dosis de Tremfya.
Informe a su médico si está embarazada, cree que pudiera estarlo o tiene intención de quedarse embarazada.
- Informe también a su médico si está dando de mamar o tiene previsto hacerlo. Usted y su médico deben decidir si puede dar de mamar o usar Tremfya.
Conducción y uso de máquinas
Es poco probable que Tremfya afecte a su capacidad para conducir y usar máquinas.
3. Cómo usar Tremfya
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o enfermero. En caso de duda, consulte a su médico, enfermero o farmacéutico.
Qué cantidad hay que administrar de Tremfya y durante cuánto tiempo
Su médico decidirá durante cuánto tiempo necesita usar Tremfya.
Psoriasis en placas
- La dosis es de 100 mg (el contenido de 1 pluma precargada) administrada mediante una inyección bajo la piel (inyección subcutánea). Su médico o enfermero probablemente le pondrán esta inyección.
- Después de la primera dosis, recibirá la siguiente dosis 4 semanas después y luego cada 8 semanas.
Artritis psoriásica
- La dosis es de 100 mg (el contenido de 1 pluma precargada) administrada en forma de inyección bajo la piel (inyección subcutánea). Su médico o enfermero probablemente le pondrán esta inyección.
- Después de la primera dosis, recibirá la siguiente dosis 4 semanas después y luego cada 8 semanas. En algunos pacientes, después de la primera dosis, Tremfya se puede administrar cada 4 semanas. Su médico decidirá con qué frecuencia puede recibir Tremfya.
Al principio, su médico o enfermero le inyectarán Tremfya. Sin embargo, puede que usted junto con su médico decidan que puede inyectarse Tremfya usted mismo en cuyo caso, usted recibirá el entrenamiento apropiado sobre cómo inyectar Tremfya. Consulte a su médico o enfermero si tiene alguna duda sobre la administración de las inyecciones. Es importante que no intente inyectarse usted mismo hasta que no le hayan enseñado su médico o enfermero.
Para saber cómo debe utilizar Tremfya, lea atentamente el prospecto de «Instrucciones de uso» antes de usarlo, que se adjunta en el envase.
Si usa más Tremfya del que debe
Si ha recibido más Tremfya del que debe o la dosis se ha administrado antes del momento prescrito, informe a su médico.
Si olvidó usar Tremfya
Si se ha olvidado de inyectar una dosis de Tremfya, informe a su médico.
Si interrumpe el tratamiento con Tremfya
No deje de utilizar Tremfya sin hablar antes con su médico. Si usted suspende el tratamiento, sus síntomas pueden reaparecer.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Informe a su médico o busque ayuda médica inmediatamente si presenta alguno de los siguientes efectos adversos:
Posible reacción alérgica grave(puede afectar hasta 1 de cada 100 personas) - los signos pueden incluir:
- dificultad para respirar o tragar
- hinchazón de la cara, los labios, la lengua o la garganta
- picor intenso de la piel, con una erupción roja o abultamientos
Otros efectos adversos
Los siguientes efectos adversos son todos de leves a moderados. Si alguno de estos efectos adversos llega a ser grave, consulte a su médico, farmacéutico o enfermero inmediatamente.
Algunos efectos adversos son muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- infecciones de las vías respiratorias
Algunos efectos adversos son frecuentes (pueden afectar hasta 1 de cada 10 personas):
- dolor de cabeza
- dolor en las articulaciones (artralgia)
- diarrea
- enrojecimiento, irritación o dolor en el lugar de inyección
- niveles aumentados en sangre de enzimas del hígado
Algunos efectos adversos son poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- reacción alérgica
- erupción cutánea
- disminución del número de un tipo de glóbulos blancos llamados neutrófilos
- infecciones por herpes simple
- infección de la piel por hongos, por ejemplo entre los dedos de los pies (p. ej., pie de atleta)
- malestar estomacal (gastroenteritis)
- habones
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto.También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Tremfya
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta de la pluma precargada y en la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar la pluma precargada en el embalaje exterior para protegerla de la luz.
Conservar en nevera (2°C–8°C). No congelar.
No agitar.
No use este medicamento si advierte que está turbio o tiene un color anormal, o contiene partículas grandes. Antes de usar el medicamento, saque el envase de la nevera y mantenga la pluma precargada en su interior hasta que alcance la temperatura ambiente esperando 30 minutos.
Este medicamento es para un único uso. Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Tremfya
- El principio activo es guselkumab. Cada pluma precargada contiene 100 mg de guselkumab en 1 ml de solución.
- Los otros componentes son histidina, monoclorhidrato de histidina monohidratado, polisorbato 80, sacarosa y agua para preparaciones inyectables.
Aspecto de Tremfya y contenido del envase
Solución inyectable (inyección). Tremfya es una solución transparente y de incolora a color amarillo claro. Se presenta en un envase de cartón que contiene una pluma precargada unidosis y como un envase múltiple conteniendo 2 (2 envases de 1) plumas precargadas unidosis. Puede que no estén comercializados todos los tamaños de envase.
Titular de la autorización de comercialización
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Bélgica
Fabricante
Janssen Biologics B.V.
Einsteinweg 101
2333CB Leiden
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Janssen‑Cilag NV Tel/Tél: +32 14 64 94 11 | Lietuva UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
???????? „??????? & ??????? ????????” ???? ???.: +359 2 489 94 00 | Luxembourg/Luxemburg Janssen‑Cilag NV Tél/Tel: +32 14 64 94 11 |
Ceská republika Janssen‑Cilag s.r.o. Tel.: +420 227 012 227 | Magyarország Janssen‑Cilag Kft. Tel.: +36 1 884 2858 |
Danmark Janssen‑Cilag A/S Tlf: +45 45 94 8282 | Malta AM MANGION LTD. Tel: +356 2397 6000 |
Deutschland Janssen‑Cilag GmbH Tel: +49 2137 955 955 | Nederland Janssen‑Cilag B.V. Tel: +31 76 711 1111 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Tel: +372 617 7410 | Norge Janssen‑Cilag AS Tlf: +47 24 12 65 00 |
Ελλ?δα Janssen‑Cilag Φαρμακευτικ? Α.Ε.Β.Ε. Tηλ: +30 210 80 90 000 | Österreich Janssen‑Cilag Pharma GmbH Tel: +43 1 610 300 |
España Janssen‑Cilag, S.A. Tel: +34 91 722 81 00 | Polska Janssen‑Cilag Polska Sp. z o.o. Tel.: +48 22 237 60 00 |
France Janssen‑Cilag Tél: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen‑Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Hrvatska Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Ísland Janssen‑Cilag AB c/o Vistor hf. Sími: +354 535 7000 | Slovenská republika Johnson & Johnson, s.r.o. Tel: +421 232 408 400 |
Italia Janssen‑Cilag SpA Tel: 800.688.777/+39 02 2510 1 | Suomi/Finland Janssen‑Cilag Oy Puh/Tel: +358 207 531 300 |
Κ?προς Βαρν?βας Χατζηπαναγ?ς Λτδ Τηλ: +357 22 207 700 | Sverige Janssen‑Cilag AB Tel: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Tel: +371 678 93561 | United Kingdom Janssen‑Cilag Ltd. Tel: +44 1 494 567 444 |
Fecha de la última revisión de este prospecto: 02/2021
La información detallada de este medicamento está disponible en la página web de la Agencia
Europea de Medicamentos: http://www.ema.europa.eu, y en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).

Si su médico decide que usted o un cuidador pueden poner las inyecciones de Tremfya en casa, debe aprender a preparar e inyectar correctamente Tremfya con la pluma precargada antes de intentar la inyección.
Lea estas instrucciones de uso antes de usar la pluma precargada de Tremfya y cada vez que renueve la receta de la pluma precargada. Podría haber nueva información. Esta guía de instrucciones no sustituye a la necesidad de hablar con su médico sobre su enfermedad o su tratamiento.
Lea también atentamente el prospecto antes de empezar a inyectarse y comente cualquier duda que tenga con su médico o enfermero.

Conservar en nevera entre 2 °C y 8 °C.
Nocongelar.
Mantenga Tremfya y todos los medicamentos fuera del alcance de los niños.
Noagite la pluma precargada en ningún momento.

Consulte a su médico cualquier pregunta que pueda tener. Para recibir ayuda adicional o compartir su experiencia, consulte la información de contacto de su representante local en el prospecto.
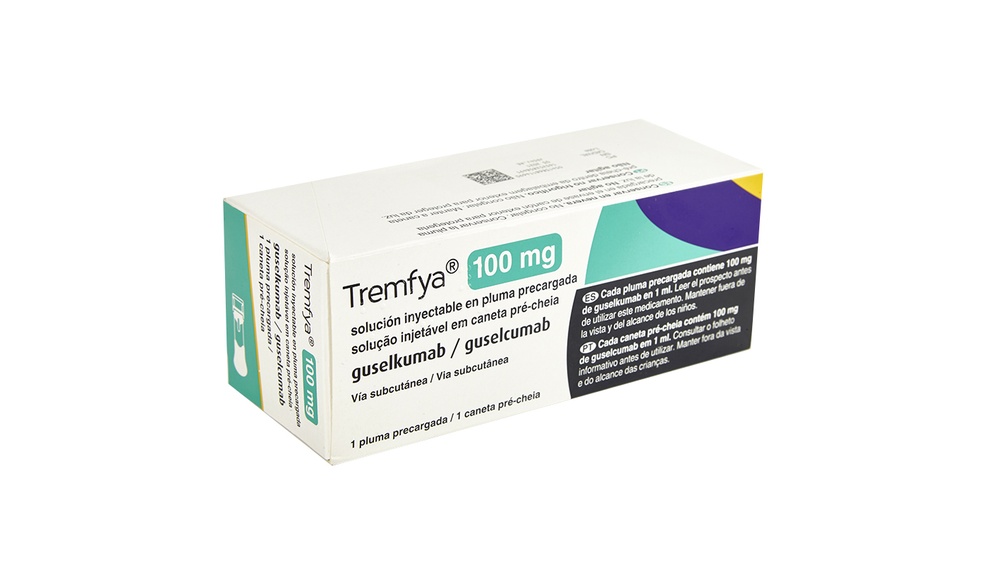
Inspeccione el envase de cartón
Saque de la nevera el envase de cartón con la pluma precargada.
Mantenga la pluma precargada en el envase de cartón y póngala en una superficie plana a temperatura ambiente durante al menos 30 minutosantes de utilizarla.
Nola caliente de ninguna otra forma.
Compruebe la fecha de caducidad(CAD) en el envase de cartón.
Nouse el medicamento después de la fecha de caducidad.
Noinyecte el medicamento si las perforaciones del envase están rotas.
Hable con su médico o farmacéutico para que le recete otra vez la pluma precargada.

Elija el lugar de inyección
Elija entre las zonas siguientes para ponerse la inyección:
- Parte delantera de los muslos(recomendado)
- Parte inferior del abdomen
Nouse el área de 5 cm alrededor del ombligo.
- Parte posterior de la parte superior del brazo (si un cuidador le pone la inyección)
Noinyecte en piel dolorida, con hematomas, roja, descamada, dura o en zonas con cicatrices o estrías.
Lávese las manos
Lávese bien las manos con agua tibia y jabón.
Limpie el lugar de inyección
Limpie la zona de inyección elegida con un algodón con alcohol y deje que se seque.
Notoque, abanique o sople en el lugar de inyección después de haberlo limpiado.

Inspeccione el líquido en la ventana
Saque la pluma precargada del envase de cartón.
Compruebe el líquido en la ventana de visión. El líquido debe ser transparente o de color amarillo claro y puede contener partículas diminutas translúcidas o blancas. También pueden verse una o más burbujas de aire.
Esto es normal.
Noinyecte si el líquido está turbio o tiene un color anormal, o contiene partículas
grandes. Si no está seguro, consulte a su médico o farmacéutico para hacerse con una nueva pluma precargada.

Gire y retire el capuchón inferior
Mantenga las manos lejos del protector de la aguja después de quitar el capuchón.
Inyecte en los 5 minutos siguientes a la retirada del capuchón de la
aguja.
Novuelva a poner el capuchón porque se podría dañar la aguja.
Nouse la pluma precargada si se cae después de quitar el capuchón.
Hable con su médico o farmacéutico para hacerse con una nueva pluma precargada.

Colóquela sobre la piel
Coloque la pluma precargada directamente sobre la piel (a unos 90 grados respecto al lugar de la inyección).

Apriete el pulsador hacia abajo
Se inyecta el medicamento al apretar. Realice estos pasos a un ritmo que le resulte cómodo.
Nolevante la pluma precargada durante la inyección. Se bloqueará el protector de la aguja y no se administrará la dosis completa.

Inyección finalizada
Se finaliza la inyección cuando se aprieta el pulsador hacia abajo en todo su recorrido, se oye un clic y ya no se puede ver el cuerpo verde.

Levante la pluma
La línea amarilla indica que se ha bloqueado el protector de la aguja.
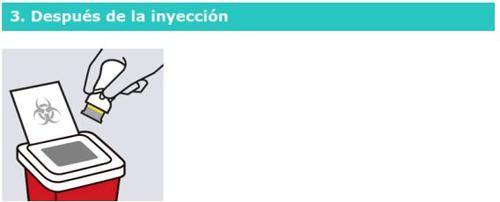
Tire la pluma precargada utilizada
Ponga la pluma precargada utilizada en un recipiente para objetos
cortopunzantes después de usarla.
Cuando el recipiente esté lleno, elimine el material según las instrucciones de su médico o enfermero.

Compruebe el lugar de inyección
Puede haber una pequeña cantidad de sangre o líquido en el lugar de inyección.
Mantenga la presión sobre la piel con una bola de algodón o una gasa hasta que la hemorragia se detenga.
Nofrote el lugar de inyección.
Si es necesario, cubra el lugar de inyección con una venda.
¡Su inyección ha finalizado!
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TREMFYA 100 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 100 mgPrincipio activo: GuselkumabFabricante: Janssen-Cilag International N.VRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 200mg/20mlPrincipio activo: GuselkumabFabricante: Janssen-Cilag International N.VRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 130 mgPrincipio activo: UstekinumabFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para TREMFYA 100 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TREMFYA 100 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






