
TOSHEDRA 35 MG JARABE EN SOBRES


Cómo usar TOSHEDRA 35 MG JARABE EN SOBRES
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Toshedra 35 mg Jarabe en sobres
Extracto seco de Hedera helix L. (hiedra)
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento porque contiene información importante para usted.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si necesita consejo o más información, consulte a su farmacéutico.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Debe consultar a un médico si empeora o si no mejora después de 7 días.
Contenido del prospecto
- Qué es Toshedra y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Toshedra
- Cómo tomar Toshedra
- Posibles efectos adversos
- Conservación de Toshedra
- Contenido del envase e información adicional
1. Qué es Toshedra y para qué se utiliza
Toshedra es un expectorante.
Toshedra es un medicamento a base de plantas utilizado como expectorante para la tos productiva que acompaña a afecciones bronquiales benignas. Facilita la eliminación de la mucosidad.
Toshedra está indicado en adultos, adolescentes y niños mayores de 6 años.
2. Qué necesita saber antes de empezar a tomar Toshedra
No tome Toshedra:
- Si es alérgico a la hiedra (Hedera helix L.), a plantas de la familia de las Araliáceas o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- No administrar a niños menores de 2 años ya que existe el riesgo de que se agraven los síntomas respiratorios.
Advertencias y precauciones:
Consulte a su médico o farmacéutico antes de empezar a tomar Toshedra.
Se debe consultar con el médico o farmacéutico en los casos de disnea (dificultad para respirar), fiebre o esputos purulentos.
No se recomienda el uso concomitante con otros antitusivos como la codeína o dextrometorfano sin previa consulta médica.
Se recomienda precaución en pacientes con gastritis o úlcera gástrica.
Este medicamento no debe administrarse a niños de 2 a 5 años ya que no puede dosificarse adecuadamente, existen otras formulaciones más adecuadas para este grupo de edad.
En caso de empeoramiento de los síntomas o si no se produjera mejoría después de 7 días de iniciar el tratamiento, éste deberá ser interrumpido y se deberá consultar con el médico.
Toma de Toshedra con otros medicamentos:
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Embarazo, lactancia y fertilidad:
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo:
No hay estudios adecuados y bien controlados en mujeres embarazadas por lo que no se recomienda su administración.
Lactancia:
No existe información del paso de los componentes de este medicamento a la leche materna, por lo que no se recomienda su administración a mujeres durante el período de lactancia.
Fertilidad:
No existen datos disponibles sobre fertilidad.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Conducción y uso de máquinas
No se han realizado estudios de los efectos sobre la capacidad para conducir y utilizar máquinas.
Toshedra contiene sorbitol.
Este medicamento contiene 1.925 mg de sorbitol en cada sobre de 5 ml (385 mg por cada ml).
El sorbitol es una fuente de fructosa. Si su médico le ha indicado que usted (o su hijo) padecen una intolerancia a ciertos azúcares, o se les ha diagnosticado intolerancia hereditaria a la fructosa (IHF), una enfermedad genética rara, en la que el paciente no puede descomponer la fructosa, consulte usted (o su hijo) con su médico antes de tomar este medicamento.
El sorbitol puede provocar malestar gastrointestinal y un ligero efecto laxante.
3. Cómo tomar Toshedra
Siga exactamente las instrucciones de administración de este medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico. En caso de duda, pregunte a su médico o farmacéutico.
La dosis recomendada es:
- Adultos y adolescentes mayores de 12 años: 5 ml de jarabe (1 sobre), 3 veces al día, (equivalente a 105 mg diarios de extracto seco de hojas de hiedra).
- Niños entre 6 y 12 años de edad:5 ml de jarabe (1 sobre), 2 veces al día, (equivalente a 70 mg diarios de extracto seco de hojas de hiedra).
Si estima que la acción de Toshedra es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Toshedra se toma por vía oral.
Para más detalles del uso de los sobres, siga los diagramas siguientes:
Presionar suavemente el sobre antes de usarlo, como se muestra.
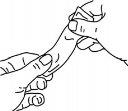
Sujetar firmemente el sobre y rasgar el sobre por donde indican las rayas.
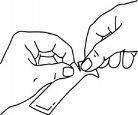
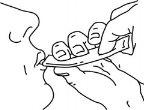
Tragar el medicamento, apretando el sobre hasta que quede vacío.
Debe consultar a un médico si empeora o no mejora después de una semana de tratamiento.
Si toma más Toshedra del que debe
En el caso de que se haya tomado más Toshedra del que debiera o en caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 915 620 420, indicando el medicamento y la cantidad ingerida.
No exceda la dosis diaria recomendada. La ingestión de cantidades significativamente más altas (más de tres veces la dosis diaria) puede provocar náuseas, vómitos y diarrea.
En este caso, debe consultar a su médico.
Si olvidó tomar Toshedra:
No tome una dosis doble para compensar la dosis olvidada.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Frecuentes (pueden afectar a entre 1 y 10 de cada 100 pacientes): se han notificado reacciones del sistema gastrointestinal como náuseas, vómitos o diarrea.
Poco frecuentes (pueden afectar a entre 1 y 10 de cada 1.000 pacientes): se han notificado reacciones alérgicas como urticaria, erupciones cutáneas, dificultad para respirar (disnea).
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.
Si nota síntomas de alergia (hipersensibilidad), interrumpa la toma de Toshedra.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Toshedra
Mantener este medicamento fuera de la vista y del alcance de los niños.
No requiere condiciones especiales de conservación.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase, después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los
medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Toshedra 35 mg jarabe en sobres:
El principio activo es: extracto seco de hoja de Hedera helix. 5 ml de Toshedra contienen 35 mg de extracto seco de hoja de Hedera helix L. (hiedra) (4-8:1), disolvente de extracción: etanol 30% (m/m). Los demás componentes son: agua purificada, sorbato de potásio, ácido cítrico, sorbitol líquido no cristalizable (E-420), goma xantán y esencia de cereza.
Aspecto del producto y contenido del envase:
Toshedra 35 mg jarabe se presenta en sobres unidosis de fácil apertura, de 5 ml cada uno, formados por una lámina compleja de aluminio (PET/Alu/PE/PET/PE).
Envase con 20 sobres.
Titular de la autorización de comercialización y responsable de la fabricación:
Kern Pharma, S.L.
Venus, 72 – Pol. Ind. Colón II
08228 Terrassa – Barcelona
España
Fecha de la última revisión de este prospecto: Noviembre 2023
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TOSHEDRA 35 MG JARABE EN SOBRESForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 7 mg/mlPrincipio activo: Hederae helicis foliumFabricante: Engelhard Arzneimittel Gmbh & Co. KgNo requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 35 mgPrincipio activo: Hederae helicis foliumFabricante: Adventia Pharma S.L.No requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 7 mg/mlPrincipio activo: Hederae helicis foliumFabricante: Adventia Pharma S.L.No requiere receta
Médicos online para TOSHEDRA 35 MG JARABE EN SOBRES
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TOSHEDRA 35 MG JARABE EN SOBRES, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















