TEZSPIRE 210 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar TEZSPIRE 210 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Tezspire 210 mg solución inyectable en pluma precargada
tezepelumab
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Tezspire y para qué se utiliza
- Qué necesita saber antes de empezar a usar Tezspire
- Cómo usar Tezspire
- Posibles efectos adversos
- Conservación de Tezspire
- Contenido del envase e información adicional
1. Qué es Tezspire y para qué se utiliza
Qué es Tezspire y cómo funciona
Tezspire contiene la sustancia activa tezepelumab, que es un anticuerpo monoclonal. Los anticuerpos son proteínas que reconocen y se unen a una sustancia diana específica en el organismo, que en el caso de tezepelumab es una proteína llamada linfopoyetina estromal tímica(TSLP). La TSLP desempeña un papel clave en la inflamación de las vías respiratorias que causa los signos y síntomas del asma. Al bloquear la acción de la TSLP, este medicamento ayuda a reducir la inflamación y los síntomas del asma.
Para qué se utiliza Tezspire
Tezspire se utiliza para tratar el asma grave en adultos y adolescentes junto con otros medicamentos para el asma (a partir de 12 años) cuando la enfermedad no está controlada con sus medicamentos actuales.
Cómo puede ayudar Tezspire
Tezspire puede disminuir la frecuencia de crisis asmáticas que experimenta, mejorar su respiración y reducir sus síntomas de asma.
2. Qué necesita saber antes de empezar a usar Tezspire
No use Tezspire
- si es alérgicoa tezepelumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Si esto le afecta a usted, o si no está seguro, consulte con su médico, farmacéutico o enfermero.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Tezspire.
- Tezspire no es un medicamento de rescate.No lo use para tratar una crisis asmática repentina.
- Si su asma no mejora, o empeoradurante el tratamiento con este medicamento, consulte con un médico o enfermero.
- Esté atento a los signos de reacciones alérgicas.Los medicamentos como Tezspire pueden potencialmente causar reacciones alérgicas graves en algunas personas. Los signos de estas reacciones pueden variar, pero pueden incluir hinchazón de la cara, lengua o boca, problemas respiratorios, ritmo cardíaco acelerado, desmayo, mareo, aturdimiento, habones y erupción cutánea. Si nota alguno de estos signos, hable con un médico o enfermero inmediatamente.
Consulte a su médico sobre cómo reconocer los primeros signos de alergia y cómo manejar las reacciones si ocurren.
- Esté atento a cualquier signo de una posible infección gravemientras esté tomando Tezspire, como por ejemplo:
- fiebre, síntomas parecidos a los de la gripe, sudores nocturnos;
- tos que no desaparece;
- piel caliente, roja y dolorida, o erupción cutánea dolorosa con ampollas.
Si nota alguno de estos signos, informe a un médico o enfermero inmediatamente.
Si ya tiene una infección grave, informe a su médicoantes de recibir Tezspire.
- Esté atento a cualquier síntoma de un problema cardíaco, como:
- dolor en el pecho;
- dificultad para respirar;
- una sensación general de malestar, enfermedad o falta de bienestar;
- sensación de mareo o desmayo.
Si nota alguno de estos síntomas, informe a un médico o enfermero inmediatamente.
- Si tiene una infección parasitariao si vive (o viaja a) una zona donde las infecciones parasitarias son frecuentes, consulte a su médico. Tezspire puede disminuir la capacidad de su cuerpo para combatir determinados tipos de infecciones parasitarias.
Niños
No administre este medicamento a niños menores de 12 años porque se desconocen la seguridad y los beneficios de este medicamento en niños de este grupo de edad.
Otros medicamentos para el asma
- No suspenda bruscamentesus otros medicamentos para el asma cuando comience a usar Tezspire. Esto es especialmente importante si toma esteroides (también llamados corticosteroides). Estos medicamentos deben suspenderse gradualmente, bajo la supervisión de su médico y en función de su respuesta a Tezspire.
Otros medicamentos y Tezspire
Informe a su médico o farmacéutico:
- si está tomando, ha tomado recientemente o pudiera tener que usar cualquier otro medicamento.
- si se ha vacunado recientemente o tiene previsto hacerlo.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de usar este medicamento.
- No use Tezspire durante el embarazo a menos que su médico se lo indique. Se desconoce si Tezspire puede afectar al feto.
- Tezspire puede pasar a la leche materna. Si está dando el pecho o planea hacerlo, contacte con su médico.
Conducción y uso de máquinas
Es poco probable que Tezspire afecte su capacidad para conducir y usar máquinas.
Tezspire contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis de 210 mg; esto es, esencialmente “exento de sodio”.
3. Cómo usar Tezspire
Use siempre este medicamento exactamente como le haya indicado su médico o farmacéutico. Consulte con su médico, farmacéutico o enfermero si no está seguro.
Adultos y adolescentes a partir de 12 años:
- La dosis recomendadaes de 210 mg (1 inyección) cada 4 semanas. Tezspire se administra como una inyección debajo de la piel (vía subcutánea).
Su médico o enfermero decidirá si puede inyectarse usted mismo o si su cuidador puede hacerlo por usted. Si es así, usted o su cuidador recibirán formación sobre la forma correcta de preparar e inyectar Tezspire.
Antes de inyectarse Tezspire usted mismo, lea cuidadosamente las “Instrucciones de uso” de Tezspire pluma precargada. Haga esto cada vez que reciba otra inyección. Puede haber nueva información.
No comparta Tezspire pluma precargada ni use una pluma más de una vez.
Si olvida usar Tezspire
- Si ha olvidado inyectarse una dosis, hágalo lo antes posible. A continuación, aplique la siguiente inyección el siguiente día programado para la inyección.
- Si no se ha dado cuenta de que olvidó una dosis hasta la hora de su próxima dosis, simplemente inyecte la siguiente dosis según lo programado. No inyecte una dosis doble para compensar la dosis olvidada.
- Si no está seguro de cuándo inyectarse Tezspire, consulte a su médico, farmacéutico o enfermero.
Si interrumpe el tratamiento con Tezspire
- No interrumpa el tratamiento con Tezspire sin consultarlo primero con su médico, farmacéutico o enfermero. Interrumpir o suspender el tratamiento con Tezspire puede provocar la reaparición de sus síntomas y crisis asmáticas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones alérgicas graves
Acuda al médico inmediatamentesi cree que puede estar teniendo una reacción alérgica. Dichas reacciones pueden suceder al cabo de horas o días después de la inyección.
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- reacciones alérgicas, incluida reacción alérgica grave (anafilaxia)
los síntomas habituales incluyen:
- hinchazón en la cara, lengua o boca
- problemas de respiración, ritmo cardíaco acelerado
- desmayo, mareo, aturdimiento
Otros efectos adversos
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- dolor de garganta
- erupción cutánea
- dolor en las articulaciones
- reacción en el lugar de la inyección (como enrojecimiento, hinchazón y dolor)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Tezspire
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No lo utilice después de la fecha de caducidad que aparece en la etiqueta y el cartonaje. La fecha de caducidad es el último día del mes que se indica.
- Conservar en nevera (entre 2ºC y 8ºC).
- Mantener la pluma precargada en el embalaje exterior para protegerla de la luz.
- Tezspire se puede conservar a temperatura ambiente (de 20ºC a 25ºC) en el embalaje exterior durante un máximo de 30 días. Una vez que Tezspire haya alcanzado la temperatura ambiente, no lo vuelva a colocar en la nevera. Si Tezspire se ha almacenado a temperatura ambiente durante más de 30 días debe desecharse de manera segura.
- No agitar, congelar ni exponer al calor.
- No utilice este medicamento si se ha caído o dañado, o si el sello de seguridad del embalaje exterior se ha roto.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Tezspire
- El principio activo es tezepelumab.
- Los demás componentes son ácido acético, L-prolina, polisorbato 80, hidróxido de sodio y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Tezspire es una solución transparente u opalescente, de incolora a amarillo claro.
Tezspire está disponible en un envase que contiene 1 pluma precargada o en un envase múltiple que contiene 3 plumas precargadas (3 envases de 1).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
AstraZeneca AB
SE 151 85 Södertälje
Suecia
Responsable de la fabricación
AstraZeneca AB
Gärtunavägen
SE-152 57 Södertälje
Suecia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien AstraZeneca S.A./N.V. Tel: +32 2 370 48 11 | Lietuva UAB AstraZeneca Lietuva Tel: +370 5 2660550 |
| Luxembourg/Luxemburg AstraZeneca S.A./N.V. Tél/Tel: +32 2 370 48 11 |
Ceská republika AstraZeneca Czech Republic s.r.o. Tel: +420 222 807 111 | Magyarország AstraZeneca Kft. Tel.: +36 1 883 6500 |
Danmark AstraZeneca A/S Tlf: +45 43 66 64 62 | Malta Associated Drug Co. Ltd Tel: +356 2277 8000 |
Deutschland AstraZeneca GmbH Tel: +49 40 809034100 | Nederland AstraZeneca BV Tel: +31 85 808 9900 |
Eesti AstraZeneca Tel: +372 6549 600 | Norge AstraZeneca AS Tlf: +47 21 00 64 00 |
Ελλáδα AstraZeneca A.E. Τηλ: +30 210 6871500 | Österreich AstraZeneca Österreich GmbH Tel: +43 1 711 31 0 |
España AstraZeneca Farmacéutica Spain, S.A. Tel: +34 91 301 91 00 | Polska AstraZeneca Pharma Poland Sp. z o.o. Tel.: +48 22 245 73 00 |
France AstraZeneca Tél: +33 1 41 29 40 00 | Portugal AstraZeneca Produtos Farmacêuticos, Lda. Tel: +351 21 434 61 00 |
Hrvatska AstraZeneca d.o.o. Tel: +385 1 4628 000 | România AstraZeneca Pharma SRL Tel: +40 21 317 60 41 |
Ireland AstraZeneca Pharmaceuticals (Ireland) DAC Tel: +353 1609 7100 | Slovenija AstraZeneca UK Limited Tel: +386 1 51 35 600 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika AstraZeneca AB, o.z. Tel: +421 2 5737 7777 |
Italia AstraZeneca S.p.A. Tel: +39 02 00704500 | Suomi/Finland AstraZeneca Oy Puh/Tel: +358 10 23 010 |
Κúπρος Αλéκτωρ Φαρµακeυτικ? Λτδ Τηλ: +357 22490305 | Sverige AstraZeneca AB Tel: +46 8 553 26 000 |
Latvija SIA AstraZeneca Latvija Tel: +371 67377100 | United Kingdom (Northern Ireland) AstraZeneca UK Ltd Tel: +44 1582 836 836 |
Fecha de la última revisión de este prospecto
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Instrucciones de uso
Tezspire 210 mg solución inyectable en pluma precargada
tezepelumab
Estas ‘Instrucciones de Uso’ contienen información sobre cómo inyectar Tezspire.
Antes de usar Tezspire pluma precargada, un profesional sanitario debe enseñarle a usted o a su cuidador cómo usarlo correctamente.
Lea estas ‘Instrucciones de uso’ antes de empezar a usar Tezspire pluma precargada y cada vez que reciba otra inyección.Puede haber nueva información. Esta información no debe sustituir a la consulta con su profesional sanitario sobre su enfermedad y su tratamiento.
Si usted o su cuidador tienen cualquier duda, consulten con su profesional sanitario.
Información importante que debe saber antes de inyectarse Tezspire
Conserve Tezspire en una nevera entre 2ºC y 8ºC en su embalaje exterior hasta que esté listo para usarlo.Tezspire puede mantenerse a temperatura ambiente entre 20ºC y 25ºC en el embalaje exterior durante un máximo de 30 días.
Una vez Tezspire haya alcanzado la temperatura ambiente, nolo vuelva a colocar en la nevera.
Tire (deseche) el Tezspire que se haya almacenado a temperatura ambiente durante más de 30 días (ver Paso 10).
No usesu Tezspire pluma precargada si: | Noagite su pluma precargada. |
| Nocomparta ni use su pluma precargada más de 1 vez. |
| |
| Noexponga su Tezspire pluma precargada al calor. |
|
Si ocurre algo de lo anterior, deseche la pluma precargada en un contenedor para objetos punzantes y use un nuevo Tezspire pluma precargada.
Cada Tezspire pluma precargada contiene 1 dosis de Tezspire que es de un solo uso.
Mantenga Tezspire pluma precargada y todos los medicamentos fuera de la vista y del alcance de los niños.
Tezspire se administra solo como una inyección debajo de la piel (subcutánea).
Su Tezspire pluma precargada
Noretire la cápsula de cierre hasta el Paso 6 de estas instrucciones cuando esté listo para inyectar Tezspire.
Antes del uso | Después del uso | ||||
Ventana de visión | |||||
|
| ||||
Etiqueta con fecha de caducidad | Medicamento líquido | Cápsula de cierre | Émbolo naranja | Protector de la aguja naranja |
Preparación para inyectar Tezspire
Paso 1 – Reúna todos los materiales
- 1 Tezspire pluma precargada de la nevera
- 1 toallita con alcohol
- 1 torunda de algodón o gasa
- 1 pequeño apósito (opcional)
- 1 contenedor para depositar objetos punzantes. Vea el Paso 10 para obtener instrucciones sobre cómo tirar (desechar) Tezspire pluma precargada usada de manera segura.
|
|
|
| |
Pluma precargada | Toallita con alcohol | Torunda de algodón o gasa | Apósito | Contenedor para depositar objetos punzantes |
Paso 2 – Prepárese para usar su Tezspire pluma precargada
Deje que Tezspire alcance una temperatura ambiente entre 20ºC y 25ºC durante aproximadamente 60 minutos o más (hasta un máximo de 30 días) antes de administrar la inyección. Mantenga la pluma precargada en el embalaje exterior para protegerla de la luz. Nocaliente la pluma precargada de ninguna otra manera. Por ejemplo, nola caliente en un microondas o agua caliente, bajo la luz solar directa o cerca de otras fuentes de calor. |
|
Novuelva a colocar Tezspire en la nevera después de que haya alcanzado la temperatura ambiente.
Tire (deseche) el Tezspire que haya estado almacenado a temperatura ambiente durante más de 30 días.
Noretire la cápsula de cierre hasta el Paso 6.
Paso 3 – Retire y compruebe la pluma precargada
Sujete el centro del cuerpo de la pluma precargada para retirar la pluma precargada de su bandeja. Compruebe si la pluma precargada está dañada. Nouse la pluma precargada si la pluma precargada está dañada. Compruebe la fecha de caducidadde la pluma precargada. Nouse la pluma precargada si la fecha de caducidad ha pasado. Observe el líquido a través de la ventana de visión.El líquido debe ser transparente y de incoloro a amarillo claro. Noinyecte Tezspire si el líquido está turbio, decolorado o contiene partículas de gran tamaño. Puede que vea pequeñas burbujas de aire en el líquido. Esto es normal. No necesita hacer nada al respecto. |
|
Fecha de caducidad |
Inyectar Tezspire
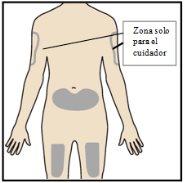
Paso 4 – Escoja un lugar de inyección
Si se está inyectando usted mismo, el lugar de inyección recomendadoes la parte delantera del muslo o la parte inferior del estómago (abdomen). Nose inyecte en el brazo. Un cuidador puede inyectarle en la parte superior del brazo, muslo o abdomen. Para cada inyección, elija un lugar diferente, separado al menos a 3 cm del lugar donde se realizó la inyección anterior. Nolo inyecte:
|
|
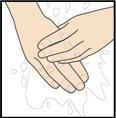
Paso 5 – Lávese las manos y limpie el lugar de la inyección
Lávese bien las manos con agua y jabón.
Limpie el lugar de la inyección con una toallita con alcohol con un movimiento circular. Deje que seque al aire.
Notoque el área limpia antes de inyectarse.
Noabanique ni sople el área limpiada.
|
|
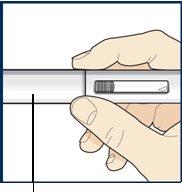
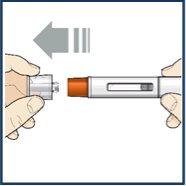
Paso 6 – Retire la cápsula de cierre
Noretire la cápsula de cierre hasta que esté listo para inyectarse. Sujete el cuerpo de la pluma con una mano y retire cuidadosamente la cápsula de cierre con la otra mano. Deje la cápsula de cierre a un lado y deséchela más tarde. El protector de la aguja naranja está ahora al descubierto. El protector de la aguja está ahí para evitar que toque la aguja. Notoque la aguja ni presione el protector de la aguja naranja con su dedo. Novuelva a colocar la cápsula de cierre en la la pluma precargada. Podría hacer que la inyección se aplicara demasiado pronto o dañar la aguja. |
|
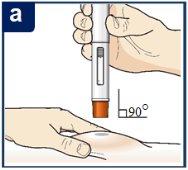
Paso 7 – Inyecte Tezspire
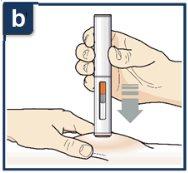
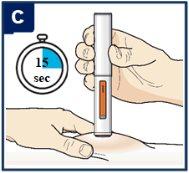
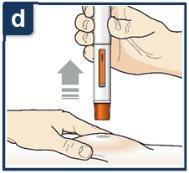
Siga las instrucciones de su profesional sanitario sobre cómo inyectar. Puede o bien pellizcar suavemente la piel en el lugar de la inyección o administrar la inyección sin pellizcar la piel. Inyecte Tezspire siguiendo los pasos de las figuras a, b, cy d. Al inyectar, oirá el primer click que indica que la inyección ha comenzado. Mantenga presionada la pluma precargada durante 15 segundos hasta que escuche el segundo click. Nocambie la posición de la pluma precargada después de que la inyeccción haya comenzado. |
|
Posición de la pluma precargada.
|
|
|
|
Presione hacia abajo firmemente hasta que deje de ver el protector de la aguja naranja.
| Mantenga firmemente presionado durante 15 segundos.
| Una vez haya completado su inyección, levante la pluma precargada hacia arriba.
|
Paso 8 – Compruebe la ventana de visión
Compruebe la ventana de visión para asegurarse de que todo el medicamento se ha inyectado. Si el émbolo naranja no ocupa por completo la ventana de visión, puede que no haya recibido la dosis completa. Si esto ocurre o tiene alguna otra preocupación, contacte a su profesional sanitario. |
| ||
Antes de la inyección |
|
| Después de la inyección |
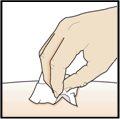
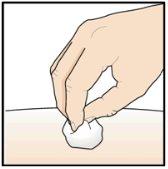
Paso 9 – Compruebe el lugar de la inyección
Puede que haya una pequeña cantidad de sangre o líquido donde se ha inyectado. Esto es normal. Presione suavemente sobre la piel con una torunda de algodón o gasa hasta que pare el sangrado. Nofrote el lugar de la inyección. Si fuera necesario, cubra el lugar de la inyección con un pequeño apósito. |
|
Desechar Tezspire
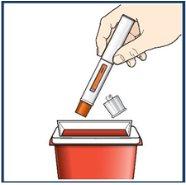
Paso 10 – Deseche la pluma precargada usada de una manera segura
Cada pluma precargada contiene una dosis única de Tezspire y no puede ser reutilizada. Novuelva a colocar la cápsula de cierre en la pluma precargada. Deposite la pluma y la cápsula de cierre usadas en un contenedor para desechar objetos punzantesinmediatamente después de su uso. Deposite los otros materiales usados en la basura doméstica. Notire la pluma precargada a la basura doméstica. |
|
Guías para desechos
Deseche el contenedor lleno según las indicaciones de su profesional sanitario o farmacéutico.
Nodeseche el contenedor para desechar objetos punzantes usado en la basura doméstica a menos que las guías de su comunidad lo permitan.
Norecicle su contenedor para desechar objetos punzantes usado.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TEZSPIRE 210 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 210 MGPrincipio activo: TezepelumabFabricante: Astrazeneca AbRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 100 mg inyectable 10 mlPrincipio activo: ReslizumabFabricante: Teva B.V.Requiere recetaForma farmacéutica: COMPRIMIDO, 250 µgPrincipio activo: roflumilastFabricante: Astrazeneca AbRequiere receta
Médicos online para TEZSPIRE 210 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TEZSPIRE 210 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes