
Temozolomida SUN 5 mg capsulas duras EFG

Cómo usar Temozolomida SUN 5 mg capsulas duras EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Temozolomida SUN 5 mg cápsulas duras EFG
Temozolomida SUN 20 mg cápsulas duras EFG
Temozolomida SUN 100 mg cápsulas duras EFG
Temozolomida SUN 140 mg cápsulas duras EFG
Temozolomida SUN 180 mg cápsulas duras EFG
Temozolomida SUN 250 mg cápsulas duras EFG
temozolomida
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Temozolomida SUN y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Temozolomida SUN
- Cómo tomar Temozolomida SUN
- Posibles efectos adversos
- Conservación de Temozolomida SUN
- Contenido del envase e información adicional
1. Qué es Temozolomida SUN y para qué se utiliza
Temozolomida SUN contiene un medicamento llamado temozolomida. Este medicamento es un agente antitumoral.
La Temozolomida SUN se utiliza para el tratamiento de determinados tipos de tumor cerebral:
- en adultos con diagnóstico reciente de glioblastoma multiforme. La Temozolomida SUN se utiliza al principio en combinación con radioterapia (fase concomitante del tratamiento) y después, por sí sola (fase de monoterapia del tratamiento).
- en niños de 3 años y mayores y adultos con glioma maligno, como el glioblastoma multiforme o astrocitoma anaplásico. Temozolomida SUN se utiliza para combatir estos tumores si reaparecen o empeoran después del tratamiento estándar.
2. Qué necesita saber antes de empezar a tomar Temozolomida SUN
No tome Temozolomida SUN si:
- es alérgico a temozolomida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si ha sufrido una reacción alérgica a la dacarbazina (un medicamento contra el cáncer en ocasiones denominado DTIC ). Los signos de reacción alérgica incluyen picores, dificultad para respirar o sibilancia, o hinchazón de la cara, labios, lengua o garganta.
- si el número de ciertos tipos de células sanguíneas, como los glóbulos blancos o plaquetas se reduce considerablemente (conocido como mielosupresión). Estas células de la sangre son importantes a la hora de combatir la infección y para que se produzca una coagulación sanguínea adecuada. Su médico le realizará un análisis de sangre para asegurarse de que tiene suficiente número de estas células antes de comenzar el tratamiento.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a tomar Temozolomida SUN
- ya que se observará de cerca si se produce el desarrollo de una forma grave de infección respiratoria llamada Pneumocystis jirovecii (neumonía PCP). Si usted ha sido diagnosticado recientemente glioblastoma multiforme, puede que le receten Temozolomida SUN durante 42 días en combinación con radioterapia. En ese caso, su médico también le recetará un medicamento para ayudarle a evitar este tipo de neumonía (PCP).
- si ha tenido alguna vez o puede que tenga ahora infección por hepatitis B, ya que Temozolomida SUN podría activar otra vez la hepatitis B, que en algunos casos puede ser mortal. Antes de iniciar el tratamiento, el médico examinará minuciosamente a los pacientes en busca de signos de esta infección.
- si tiene un recuento bajo de glóbulos rojos (anemia), de glóbulos blancos y plaquetas, o problemas de coagulación de la sangre antes de iniciar el tratamiento, o si estos problemas se desarrollan durante el tratamiento. Se le realizarán análisis de sangre con frecuencia durante el tratamiento para controlar los efectos secundarios de Temozolomida SUN en sus células sanguíneas. Su médico puede decidir reducirle la dosis, interrumpir, suspender o modificar su tratamiento. También puede necesitar otros tratamientos. En algunos casos, puede ser necesario interrumpir el tratamiento con Temozolomida SUN.
- ya que puede haber un pequeño riesgo de que se produzcan otros cambios en las células sanguíneas, incluyendo leucemia.
- si tiene náuseas (malestar) o vómitos, efectos secundarios muy comunes de la Temozolomida SUN, (consulte el apartado 4) su médico puede recetarle un medicamento (antiemético) para ayudar a prevenir el vómito.
- Si vomita frecuentemente antes o durante el tratamiento, consulte a su médico cuál es el mejor momento para tomar Temozolomida SUN hasta que los vómitos estén controlados. Si vomita después de tomar el medicamento, no tome una segunda dosis el mismo día.
- si tiene fiebre o síntomas de una infección, póngase en contacto con su médico inmediatamente.
- si es mayor de 70 años, podría ser más propenso a sufrir infecciones, sangrado o moratones.
- si tiene problemas de hígado o riñón, puede ser necesario ajustar su dosis de Temozolomida SUN.
Niños y adolescentes
No de este medicamento a niños menores de 3 años, ya que su efecto en esta edad no ha sido estudiado. Se dispone de información limitada en pacientes mayores de 3 años que han tomado Temozolomida SUN.
Otros medicamentos Temozolomida SUN
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento, ya que no debe recibir tratamiento con Temozolomida SUN durante el embarazo salvo que su médico se lo indique claramente.
Se recomiendan métodos anticonceptivos efectivos en las pacientes que puedan quedarse embarazadas durante el tratamiento con Temozolomida SUN y durante al menos 6 meses después de completar el tratamiento.
Debe interrumpir la lactancia durante el tratamiento con Temozolomida SUN.
Fertilidad masculina
Temozolomida SUN puede causar infertilidad permanente. Los pacientes varones deben usar un método anticonceptivo efectivo y no dejar embarazada a su pareja durante al menos 3 meses después de finalizar el tratamiento. Se recomienda consultar acerca de la conservación del esperma antes del tratamiento.
Conducción y uso de máquinas
Temozolomida SUN puede provocarle cansancio o somnolencia. Si es así, no conduzca ni utilice herramientas o máquinas, ni monte en bicicleta hasta ver cómo le afecta a usted este medicamento (ver sección 4).
Temozolomida SUN contiene lactosa
Temozolomida SUN contiene lactosa (un tipo de azúcar). Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
3. Cómo tomar Temozolomida SUN
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Dosis y duración del tratamiento
Su médico decidirá la dosis de Temozolomida SUN que necesita. La dosis dependerá de su tamaño (altura y peso) y de si usted tiene un tumor recurrente y ha recibido tratamiento con quimioterapia anteriormente.
Puede que le receten otros medicamentos (antieméticos) para tomar antes y/o después de Temozolomida SUN con el fin de prevenir o controlar las náuseas y vómitos.
Pacientes con diagnóstico reciente de glioblastoma multiforme
Si le han diagnosticado recientemente, el tratamiento se realizará en dos fases:
- en primer lugar, tratamiento con radioterapia (fase concomitante)
- seguido de tratamiento con Temozolomida SUN solamente (fase de monoterapia).
Durante la fase concomitante, su médico le recetará una dosis de Temozolomida SUN de 75 mg/m2 (dosis habitual). Tendrá que tomar esta dosis cada día durante 42 a 49 días en combinación con radioterapia. La dosis de Temozolomida SUN puede reducirse o suspenderse dependiendo de los resultados de los análisis de sangre y de cómo reacciona al medicamento durante la fase concomitante.
Una vez que termine la radioterapia, se suspenderá el tratamiento durante 4 semanas. Esto le ayudará a su cuerpo a recuperarse.
A continuación, iniciará la fase de monoterapia.
Durante la fase de monoterapia, la dosis y la forma de tomar Temozolomida SUN pueden variar. Su médico decidirá la dosis exacta que usted necesita. Puede tener que someterse hasta seis períodos de tratamiento (ciclos). Cada ciclo dura 28 días. La primera dosis será de 150 mg/m2. Deberá tomar la nueva dosis de Temozolomida SUN una vez al día durante los primeros 5 días ("días de dosificación") de cada ciclo. A continuación, deberá permanecer 23 días sin tomar Temozolomida SUN. Esto supone un ciclo de tratamiento de 28 días.
Después del día 28, se comenzará el próximo ciclo. Deberá volver a tomar Temozolomida SUN una vez al día durante 5 días, seguido de 23 días sin tomarla. La dosis de Temozolomida SUN puede ajustarse, reducirse o suspenderse dependiendo de los resultados de los análisis de sangre y de cómo reacciona al medicamento durante cada ciclo de tratamiento.
Pacientes con tumores recurrentes o que hayan empeorado (gliomas malignos, como glioblastoma multiforme o astrocitoma anaplásico) que sólo toman Temozolomida SUN.
Un ciclo de tratamiento con Temozolomida SUN dura 28 días.
Deberá tomar Temozolomida SUN sólo una vez al día durante los primeros 5 días. Esta dosis diaria dependerá de si ha recibido quimioterapia anteriormente.
Si no ha recibido tratamiento con quimioterapia anteriormente, su primera dosis de Temozolomida SUN será de 200 mg/m2 una vez al día durante los primeros 5 días. Si ha recibido tratamiento con quimioterapia anteriormente, su primera dosis de Temozolomida SUN será de 150 mg/m2 una vez al día durante los primeros 5 días. A continuación, deberá permanecer 23 días sin tomar Temozolomida SUN. Esto supone un ciclo de tratamiento de 28 días.
Después del día 28, se comenzará el próximo ciclo. Deberá volver a tomar Temozolomida SUN una vez al día durante 5 días, seguido de 23 días sin tomarla.
Antes de cada nuevo ciclo de tratamiento, se le realizarán análisis de sangre para comprobar si se debe ajustar la dosis de Temozolomida SUN. Dependiendo de los resultados de los análisis de sangre, su médico puede ajustar la dosis para el próximo ciclo.
Cómo tomar Temozolomida SUN
Tome la dosis que le han recetado de Temozolomida SUN una vez al día, preferiblemente a la misma hora cada día.
Tome las cápsulas con el estómago vacío, por ejemplo, al menos una hora antes de desayunar. Trague la(s) cápsula(s) entera(s) con un vaso de agua. No abra, aplaste, ni mastique las cápsulas. Si una cápsula se rompiera, evite el contacto del polvo con la piel, los ojos o la nariz. Si accidentalmente le entra en los ojos o en la nariz, lave la zona con agua.
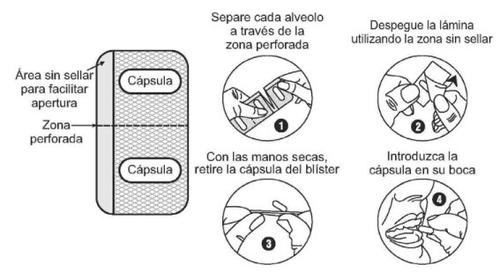
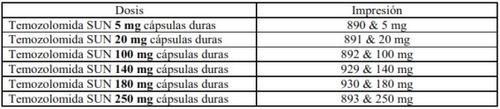
Dependiendo de la dosis que le hayan prescrito, puede tener que tomar más de una cápsula a la vez. Puede tener que tomar diferentes cantidades de principio activo para alcanzar la dosis necesaria. El marcado de la cápsula es diferente para cada dosis (ver tabla abajo).
Se debe asegurar que comprende perfectamente y recuerda lo siguiente:
- el número de cápsulas que debe tomar cada día. Pida a su médico o farmacéutico que se lo escriban (incluyendo la impresión que aparece en las cápsulas)
- qué días debe tomar el medicamento.
Consulte la dosis con su médico cada vez que inicie un ciclo nuevo, ya que puede ser diferente que la del último ciclo.
Tome Temozolomida SUN exactamente como su médico le haya indicado. Es muy importante que consulte con su médico o farmacéutico si no está seguro. Equivocarse en la forma de tomar este medicamento puede tener consecuencias graves para la salud.
Si usted toma más Temozolomida SUN del que debe
Si toma accidentalmente más cápsulas de Temozolomida SUN de las que debiera, póngase en contacto con su médico o farmacéutico o enfermero inmediatamente.
Si olvidó tomar Temozolomida SUN
Tome la dosis que olvidó lo antes posible ese mismo día. Si ha pasado un día entero, consulte con su médico. No tome una dosis doble para compensar la dosis olvidada, a menos que su médico le diga que lo haga.
Si tiene alguna duda sobre el uso de este medicamento, consulte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Consulte a su médico inmediatamente si tiene alguno de los siguientes efectos secundarios:
- reacción alérgica grave con hipersensibilidad (urticaria, sibilancias o dificultad para respirar)
- sangrado incontrolable
- ataques (convulsiones)
- fiebre
- escalofríos
- dolor de cabeza fuerte que no desaparece.
El tratamiento con Temozolomida SUN puede provocar una disminución en el número de determinados tipos de células sanguíneas. Esto puede hacerle más propenso a sufrir hematomas o sangrado, anemia (escasez de glóbulos rojos), fiebre, así como disminuir su resistencia a las infecciones. La disminución de células sanguíneas suele producirse durante un período de tiempo breve. En algunos casos, puede prolongarse y provocar un tipo de anemia muy fuerte (anemia aplásica). Su médico le realizará análisis de sangre regularmente con el fin de detectar cualquier cambio que se pueda producir y decidir si necesita un tratamiento específico. En algunos casos, se puede disminuir la dosis o suspender el tratamiento con Temozolomida SUN.
A continuación se enumeran otros efectos adversos que se han notificado:
Efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 personas) son:
- pérdida de apetito, dificultad para hablar, dolor de cabeza
- vómitos, náuseas, diarrea, estreñimiento
- erupción cutánea, pérdida de pelo
- cansancio.
Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 personas) son:
- infecciones, infecciones orales, infección de heridas
- número de células sanguíneas reducido (neutropenia, linfopenia, trombocitopenia)
- reacción alérgica
- aumento de azúcar en sangre
- alteraciones de la memoria, depresión, ansiedad, confusión, incapacidad para dormir o permanecer dormido
- alteración de la coordinación y del equilibrio
- dificultad para concentrarse, cambios en el estado mental o en el estado de alerta, sensación de hormigueo
- mareos, alteración de las sensaciones, hormigueo, temblores, gusto anormal
- pérdida parcial de la visión, visión anormal, visión doble, ojos secos o dolorosos
- sordera, zumbido en los oídos, dolor de oídos
- coágulo de sangre en los pulmones o las piernas, presión arterial alta
- neumonía, falta de aliento, bronquitis, tos, inflamación de las fosas nasales
- dolor de estómago o abdominal, malestar/acidez de estómago, dificultad para tragar
- piel seca, picor
- daño muscular, debilidad muscular, dolores y molestias musculares
- dolor de las articulaciones, dolor de espalda
- micción frecuente, dificultad para retener la orina
- fiebre, síntomas parecidos a los de la gripe, dolor, malestar, resfriado o gripe
- retención de líquidos, piernas hinchadas
- elevación de las enzimas hepáticas
- pérdida de peso, aumento de peso
- lesión por radiación.
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas) son:
- infecciones cerebrales (meningoencefalitis herpética), incluidos casos mortales
- infecciones nuevas o reactivadas por citomegalovirus
- infecciones reactivadas por el virus de la hepatitis B
- cánceres secundarios, incluida la leucemia
- reducción de los recuentos de glóbulos rojos (pancitopenia, anemia, leucopenia)
- manchas rojas debajo de la piel
- diabetes insípida (los síntomas incluyen aumento de la micción y sensación de sed), bajo nivel de potasio en la sangre
- cambios de humor, alucinaciones
- parálisis parcial, cambio en el sentido del olfato
- discapacidad auditiva, infección del oído medio
- palpitaciones (cuando puede sentir los latidos de su corazón), sofocos
- estómago hinchado, dificultad para controlar las evacuaciones intestinales, hemorroides, sequedad de boca
- hepatitis y lesión en el hígado (incluyendo insuficiencia hepática mortal), colestasis, aumento de la bilirrubina
- ampollas en el cuerpo o en la boca, descamación de la piel, erupción cutánea, enrojecimiento doloroso de la piel, erupción cutánea grave con hinchazón de la piel (incluyendo las palmas de las manos y las plantas de los pies)
- aumento de la sensibilidad a la luz solar, urticaria (ronchas), aumento de la sudoración, cambios en el color de la piel
- dificultad para orinar
- sangrado vaginal, irritación vaginal, períodos menstruales ausentes o intensos, dolor en las mamas, impotencia sexual
- escalofríos, hinchazón de la cara, decoloración de la lengua, sed, trastorno de los dientes.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Temozolomida SUN
Mantener este medicamento fuera de la vista y del alcance de los niños, preferiblemente en un armario cerrado con llave. La ingestión accidental puede provocar la muerte en niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y el envase. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25°C.
Consulte a su farmacéutico si observa cualquier cambio en el aspecto de las cápsulas.
Los medicamentos no se deben tirar por el desagüe ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Temozolomida SUN
El principio activo es temozolomida.
Temozolomida SUN 5 mg cápsulas duras: Cada cápsula dura contiene 5 mg de temozolomida.
Temozolomida SUN 20 mg cápsulas duras: Cada cápsula dura contiene 20 mg de temozolomida.
Temozolomida SUN 100 mg cápsulas duras: Cada cápsula dura contiene 100 mg de temozolomida.
Temozolomida SUN 140 mg cápsulas duras: Cada cápsula dura contiene 140 mg de temozolomida.
Temozolomida SUN 180 mg cápsulas duras: Cada cápsula dura contiene 180 mg de temozolomida.
Temozolomida SUN 250 mg cápsulas duras: Cada cápsula dura contiene 250 mg de temozolomida.
- Los demás componentes son:
contenido de las cápsula:lactosa, almidón glicolato de sodio (Tipo B), ácido tartárico, ácido esteárico (ver sección 2 “Temozolomida SUN contiene lactosa”)
cubierta de la cápsula:gelatina, dióxido de titanio (E171), laurilsulfato de sodio
tinta de impresión:
Temozolomida SUN 5 mg cápsulas duras: goma laca shellac, propilenglicol, óxido de hierro amarillo (E172), azul nº1/Laca de aluminio azul brillante FCF (E133).
Temozolomida SUN 20 mg cápsulas duras: goma laca shellac, propilenglicol, óxido de hierro amarillo (E172).
Temozolomida SUN 100 mg cápsulas duras: goma laca shellac, propilenglicol, óxido de hierro rojo (E172), oxido de hierro amarillo (E172), dióxido de titanio (E171).
Temozolomida SUN 140 mg cápsulas duras: goma laca shellac, propilenglicol, dióxido de titanio (E171), azul nº1/Laca de aluminio azul brillante FCF (E133).
Temozolomida SUN 180 mg cápsulas duras: goma laca shellac, propilenglicol, óxido de hierro rojo (E172).
Temozolomida SUN 250 mg cápsulas duras: goma laca shellac, propilenglicol, óxido de hierro negro (E172).
Aspecto de Temozolomida SUN y contenido del envase
5 mg cápsulas duras
Temozolomida SUN 5 mg cápsulas duras de gelatina tienen una tapa y un cuerpo de color blanco opaco y están impresas con tinta verde. La tapa lleva impreso ‘890’. El cuerpo lleva impreso ‘5 mg’ y dos rayas.
20 mg cápsulas duras
Temozolomida SUN 20 mg cápsulas duras de gelatina tienen una tapa y un cuerpo de color blanco opaco y están impresas con tinta amarilla. La tapa lleva impreso ‘891’. El cuerpo lleva impreso ‘20 mg’ y dos rayas.
100 mg cápsulas duras
Temozolomida SUN 100 mg cápsulas duras de gelatina tienen una tapa y un cuerpo de color blanco opaco y están impresas con tinta rosa. La tapa lleva impreso ‘892’. El cuerpo lleva impreso ‘100 mg’ y dos rayas.
140 mg cápsulas duras
Temozolomida SUN 140 mg cápsulas duras de gelatina tienen una tapa y un cuerpo de color blanco opaco y están impresas con tinta azul. La tapa lleva impreso ‘929’. El cuerpo lleva impreso ‘140 mg’ y dos rayas.
180 mg cápsulas duras
Temozolomida SUN 180 mg cápsulas duras de gelatina tienen una tapa y un cuerpo de color blanco opaco y están impresas con tinta roja. La tapa lleva impreso ‘930’. El cuerpo lleva impreso ‘180 mg’ y dos rayas.
250 mg cápsulas duras
Temozolomida SUN 250 mg cápsulas duras de gelatina tienen una tapa y un cuerpo de color blanco opaco y están impresas con tinta negra. La tapa lleva impreso ‘893’. El cuerpo lleva impreso ‘250 mg’ y dos rayas.
Las cápsulas duras están disponibles en blísters que contienen 5 cápsulas. Para las presentaciones de 20 cápsulas se incluirán 4 blísters de 5 cápsulas en una caja.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien/Ceská republika/
Danmark/Eesti/Ελλ?δα/Hrvatska/Ireland/Ísland/
Κ?προς/Latvija/Lietuva/Luxembourg/Luxemburg/Magyarország/
Malta/Nederland/Norge/Österreich/Portugal/
Slovenija/Slovenská republika/Suomi/Finland/Sverige
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Nederland/Pays-Bas/Niederlande/???????????/Nizozemsko/
Nederlandene/Holland/Ολλανδ?α/Nizozemska/The Netherlands/Holland/
Ολλανδ?α/Niderlande/Nyderlandai/Pays-Bas/Niederlande/Hollandia/
L-Olanda/Nederland/Nederland/Niederlande/Países Baixos/
Nizozemska/Holandsko/Alankomaat/Nederländerna/Nederländerna
Tel./???./tlf./τηλ./Sími/τηλ./Tlf./Puh./
+31 (0)23 568 5501
Deutschland
Sun Pharmaceuticals Germany GmbH
Hemmelrather Weg 201
51377 Leverkusen
Deutschland
Tel. +49 214 403 990
España
Sun Pharma Laboratorios S.L.
Rambla de Catalunya, 53
08007 Barcelona
España
Tel. +34 93 342 78 90
France
Ranbaxy Pharmacie Generiques
11-15, Quai de Dion Bouton
92800 Puteaux
France
Tel. +33 1 41 44 44 50
Italia
Sun Pharma Italia Srl
Viale Giulio Richard, 1
20143 – Milano
Italia
Tel. +39 02 33 49 07 93
Polska
Ranbaxy (Poland) Sp. Z o. o.
ul. Kubickiego 11
02-954 Warszawa
Polska
Tel. +48 22 642 07 75
România
Terapia S.A.
Str. Fabricii nr 124
Cluj-Napoca, Judetul Cluj
România
Tel. +40 (264) 501 500
United Kingdom
Ranbaxy UK Ltd
a Sun Pharma Company
Millington Road 11
Hyde Park, Hayes 3
5th Floor
UB3 4AZ HAYES
United Kingdom
Tel. +44 (0) 208 848 8688
Fecha de la última revisión de este prospecto:Marzo 2022
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a Temozolomida SUN 5 mg capsulas duras EFGForma farmacéutica: CAPSULA, 100 mgPrincipio activo: TemozolomidaFabricante: Merck Sharp & Dohme B.V.Requiere recetaForma farmacéutica: CAPSULA, 100 mgPrincipio activo: TemozolomidaFabricante: Merck Sharp & Dohme B.V.Requiere recetaForma farmacéutica: CAPSULA, 140 mgPrincipio activo: TemozolomidaFabricante: Merck Sharp & Dohme B.V.Requiere receta
Médicos online para Temozolomida SUN 5 mg capsulas duras EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de Temozolomida SUN 5 mg capsulas duras EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






