
TEMOZOLOMIDA ACCORD 20 mg CAPSULAS DURAS EFG

Cómo usar TEMOZOLOMIDA ACCORD 20 mg CAPSULAS DURAS EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Temozolomida Accord 5 mg cápsulas duras
Temozolomida Accord 20 mg cápsulas duras
Temozolomida Accord 100 mg cápsulas duras
Temozolomida Accord 140 mg cápsulas duras
Temozolomida Accord 180 mg cápsulas duras
Temozolomida Accord 250 mg cápsulas duras
temozolomida
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos , consulte a su médico o farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Temozolomida Accord y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Temozolomida Accord
- Cómo tomar Temozolomida Accord
- Posibles efectos adversos
- Conservación de Temozolomida Accord
- Contenido del envase e información adicional
1. Qué es Temozolomida Accord y para qué se utiliza
La temozolomida es un medicamento contra el cáncer
Temozolomida Accord cápsulas se toman para el tratamiento de formas específicas de tumores cerebrales:
- Adultos con formas específicas de tumores cerebrales de nuevo diagnóstico (glioblastoma multiforme).
- Temozolomida se usa primero con radioterapia (fase concomitante del tratamiento) y después de esto, sola (fase de monoterapia del tratamiento)
- en niños de 3 años y mayores y adultos con formas específicas de tumores cerebrales (p. ej.: glioblastoma multiforme o astrocitoma anaplásico) si reaparecen o se extienden después del tratamiento estándar.
2. Qué necesita saber antes de empezar a tomar Temozolomida Accord
No tome Temozolomida Accord
- si es alérgico a temozolomida o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si ha tenido una reacción alérgica a otro medicamento para el cáncer llamado dacarbazina. Los signos de las reacciones alérgicas incluyen picores, falta de respiración o aliento, hinchazón en cara, labios, lengua y garganta
- si tiene un número reducido de células sanguíneas, tales como el recuento de células blancas o de plaquetas Estas células de la sangre son importantes para la lucha contra la infección y para la coagulación adecuada. Su médico le realizará análisis de sangre para asegurarse de que tiene suficiente cantidad de estas células antes de comenzar el tratamiento.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a tomar Temozolomida Accord
- su médico vigilará el posible desarrollo de una forma grave de infección en el pecho llamada neumonía por Pneumocystis carinii jirovecii(PCP). Si usted es un paciente de nuevo diagnóstico de glioblastoma multiforme es posible que se le administre temozolomida durante 42 días en combinación con radioterapia. En este caso, su médico también le recetará un medicamento para ayudarle a prevenir este tipo de neumonía (PCP).
- si ha tenido alguna vez o puede que tenga ahora infección por hepatitis B, ya que temozolomida Accord podría activar otra vez la hepatitis B, que en algunos casos puede ser mortal. Antes de iniciar el tratamiento, el médico examinará minuciosamente a los pacientes en busca de signos de esta infección.
- si tiene anemia, recuentos sanguíneos bajos (p.ej. de glóbulos blancos o plaquetas), o problemas de coagulación de la sangre antes del tratamiento o los desarrolla durante el tratamiento. El médico puede necesitar reducirle la dosis del medicamento, interrumpir su tratamiento o puede ser que necesite otro tratamiento. El médico decidirá si es necesario realizar algún cambio en su tratamiento. En algunos casos, puede ser necesario dejar el tratamiento con temozolomida. Le harán análisis de sangre periódicamente para controlar su enfermedad. Si tiene fiebre o síntomas de infección, póngase en contacto con su médico inmediatamente.
- Es posible que tenga un pequeño riesgo de otros cambios en las células sanguíneas, incluyendo leucemia.
- si tiene nauseas o vómitos, lo cual es una reacción adversa muy frecuente de temozolomida (ver sección 4). Si vomita frecuentemente antes o durante el tratamiento, pregunte a su médico sobre los medicamentos que ayudan a prevenir los vómitos o a controlar los vómitos y la mejor hora para tomar temozolomida hasta que controle los vómitos. Si vomita después de tomar la dosis, no tome una segunda dosis ese mismo día.
- si presenta fiebre o síntomas de una infección, póngase en contacto con su médico inmediatamente.
- si tiene más de 70 años. Los pacientes de edad avanzada son más propensos a las infecciones, moratones y hemorragias.
- si tiene problemas de hígado o riñón, ya que podrá ser necesario ajustarle la dosis.
Niños y adolescentes
No de este medicamento a niños menores de 3 años, ya que su efecto en esta edad no ha sido estudiado. Se dispone de información limitada en pacientes mayores de 3 años que han tomado Temozolomida.
Toma de Temozolomida Accord con otros medicamentos
Informe a su médico o farmacéutico si está tomando o ha tomado recientemente recientemente o podría tener que tomar cualquier otro medicamento.
Embarazo, lactancia y fertilidad.
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento, ya que no debe ser tratada con Temozolomida Accord durante el embarazo a menos que sea claramente indicado por su médico.
Se deben usar métodos anticonceptivos efectivos en pacientes mujeres con capacidad de gestación durante el tratamiento con Temozolomida Accord y durante al menos durante 6 meses después de completar el tratamiento.
No debe dar el pecho mientras dure el tratamiento con Temozolomida Accord.
Fertilidad masculina
Temozolomida puede causar infertilidad permanente. Los pacientes varones deben usar un método anticonceptivo efectivo y no dejar embarazada a su pareja durante al menos 3 meses después de finalizar el tratamiento. Se recomienda consultar acerca de la conservación del esperma antes del tratamiento.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Conducción y uso de máquinas
Cuando tome temozolomida se puede sentir cansado o soñoliento. En este caso, no conduzca ni utilice herramientas o máquinas, ni monte en bicicleta hasta ver cómo le afecta a usted este medicamento (ver sección 4).
Temozolomida Accord contiene lactosa
Las cápsulas contienen lactosa (un tipo de azúcar). Si su médico le ha dicho que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Temozolomida Accord contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1mmol) por cápsula; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Temozolomida Accord
Siga exactamente las instrucciones de administración de Temozolomida Accord indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cómo abrir el sobre
Abra el sobre doblando y rasgándolo por la línea del pliegue con la muesca en la esquina del sobre.
Únicamente los especialistas con experiencia en tumores cerebrales deben prescribir temozolomida
Dosis y duración del tratamiento.
Su médico decidirá cuál es la dosis correcta de temozolomida en función de su complexión (altura y peso) y de si ha recibido previamente tratamiento con quimioterapia. Le podrá administrar otros medicamentos para tomar antes y/o después de la temozolomida para evitar o controlar los vómitos.
Tome la dosis prescrita de Temozolomida Accord una vez al día, con el estómago vacío; por ejemplo, al menos una hora antes del desayuno. Tome la(s) cápsula(s) entera(s) con un vaso de agua. No debe abrir, aplastar, ni masticar las cápsulas.
Si una cápsula está deteriorada, evite el contacto del polvo con la piel, ojos o nariz. Evite inhalar el polvo. Si accidentalmente penetra algo en sus ojos o nariz, lávese la zona con agua.
Si toma Temozolomida Accord en combinación con radioterapia (pacientes de nuevo diagnóstico):
Al mismo tiempo que recibe radioterapia, su médico comenzará el tratamiento con temozolomida a una dosis de 75 mg/m² y la dosis real que tome dependerá de su altura y peso. Tomará esta dosis cada día durante 42 días (hasta un máximo de 49 días) en combinación con radioterapia. En función de los recuentos sanguíneos y de cómo tolere la temozolomida, se podrá posponer o suspender la dosis.
Una vez acabada la radioterapia, interrumpirá el tratamiento durante 4 semanas para darle a su organismo la oportunidad de recuperarse.
Podrá haber hasta 6 ciclos de tratamiento y cada uno dura 28 días. Tomará su nueva dosis de las cápsulas de temozolomida en un principio a una dosis de 150 mg/m² una vez al día durante los primeros 5 días de cada ciclo ("días de administración"), seguidos de 23 días sin temozolomida. Esto suma en total un ciclo de tratamiento de 28 días.
Después del día 28, comenzará el siguiente ciclo, en el que volverá a tomar el medicamento una vez al día durante 5 días, seguidos de 23 días sin temozolomida. En función de los recuentos sanguíneos y de cómo tolere la temozolomida durante cada ciclo de tratamiento, se podrá ajustar, posponer o suspender la dosis.
Si toma Temozolomida Accord cápsulas sola (sin radioterapia):
Un ciclo de tratamiento con Temozolomida Accord comprende 28 días. Tomará las cápsulas una vez al día durante los primeros 5 días (“días de administración”), seguidos de 23 días sin temozolomida. Esto suma en total un ciclo de tratamiento de 28 días.
Después del día 28, comenzará el siguiente ciclo, en el que volverá a tomar el medicamento una vez al día durante 5 días, seguidos de 23 días sin temozolomida. Antes de cada ciclo de tratamiento nuevo, le realizarán un análisis de sangre para ver si es necesario ajustar la dosis de temozolomida.
Si no ha recibido previamente tratamiento con quimioterapia, su primera dosis de temozolomida será de 200 mg/m² una vez al día durante los primeros 5 días ("días de administración"), seguidos de 23 días sin temozolomida. Si ha recibido previamente tratamiento con quimioterapia, su primera dosis de temozolomida será de 150 mg/m² una vez al día durante los primeros 5 días (días de administración"), seguidos de 23 días sin temozolomida.
En función de los resultados de los análisis de sangre, su médico puede ajustarle la dosis para el siguiente ciclo.
Cada vez que comience un nuevo ciclo de tratamiento, asegúrese de que entiende exactamente cuántas cápsulas de cada concentración debe tomar cada día y cuántos días debe tomar esta dosis.
Todos los pacientes
La temozolomida se presenta en cápsulas de diferentes concentraciones (se muestra en la etiqueta de la caja en mg). Cada concentración tiene una tapa de diferente color. Dependiendo de la dosis de temozolomida que su médico le prescriba, puede que tenga que tomar varias cápsulas cada día de administración del ciclo de tratamiento.
- Asegúrese de que entiende exactamente cuántas cápsulas de cada concentración debe tomar. Pida a su médico o farmacéutico que le escriban el número de cápsulas de cada concentración (incluso el color) que tiene que tomar cada día de administración.
- Asegúrese de que sabe exactamente cuales son sus días de administración.
- Asegúrese de que repasa la dosis con su médico cada vez que comienza un nuevo ciclo. Algunas veces la dosis o la mezcla de cápsulas que necesita tomar puede ser diferente a la del ciclo anterior.
- Una vez que tenga el medicamento en casa, si tiene dudas o necesita aclarar algo sobre cómo tomar la dosis, llame para que le vuelvan a explicar antes de comenzar el ciclo de tratamiento. Los errores en la toma de este medicamento pueden tener consecuencias graves para su salud.
Si toma más Temozolomida Accord del que debe
Si toma accidentalmente más cápsulas de las indicadas, consulte a su médico, farmacéutico o enfermero inmediatamente.
Si olvidó tomar Temozolomida Accord
Tome la dosis que ha olvidado lo antes posible durante el mismo día. Si ha transcurrido un día entero, consulte con su médico. No tome una dosis doble para compensar la dosis olvidada, a menos que su médico le indique que lo haga.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los pacientes que toman temozolomida en combinación con radioterapia pueden presentar efectos adversos diferentes a las personas que toman temozolomida sola.
Póngase en contacto con su médico inmediatamente si presenta lo siguiente:
- una reacción alérgica grave (hipersensibilidad) (urticaria, respiración sibilante u otra dificultad respiratoria),
- hemorragia incontrolada,
- convulsiones,
- fiebre,
- escalofríos,
- dolor de cabeza intenso que no desaparece.
El tratamiento con temozolomida puede producir la reducción de ciertos tipos de células sanguíneas. Esto puede hacer que aumente la probabilidad de presentar moratones o hemorragia, anemia (falta de células rojas sanguíneas), fiebre y/o disminución de la resistencia a las infecciones. La disminución del recuento de células sanguíneas es generalmente pasajera, pero en algunos casos se puede prolongar y producir una forma muy grave de anemia (anemia aplásica). Su médico le realizará con frecuencia análisis de sangre para detectar cualquier cambio, y decidirá si necesita un tratamiento específico. En algunos casos, reducirá o suspenderá la dosis de temozolomida.
Otros efectos adversos que se han reportado son:
Efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Pérdida de apetito, dificultad en el habla, dolor de cabeza
- Vómitos, náuseas, diarrea, estreñimiento
- Erupción, pérdida de cabello
- Cansancio
Efectos adversos frecuentes (puede afectar hasta 1 de cada 10 personas):
- Infecciones, infecciones orales, infección de heridas
- Cantidad de células sanguíneas reducida (neutropenia, limfopenia, trombocitopenia)
- Reacción alergica
- Aumento de azúcar en sangre
- Alteraciones en la memoria, depresión, ansiedad, confusión, incapacidad para dormirse o permanecer dormido
- Alteración en el equilibrio y coordinación
- Dificultad para concentrarse, cambios en el estado mental o falta de alerta, olvidos
- Mareos, sensaciones alteradas, hormigueo, temblores, gusto anormal
- Pérdida parcial de visión, visión anormal, visión doble, sequedad o dolor en los ojos
- Sordera, ruidos en el oído, dolor de oído
- Coágulo en pulmón o piernas, presión arterial alta
- Pneumonía, dificultad respiratoria, bronquitis, tos, sinusitis
- Dolor de estómago o abdominal, ardor de estómago, dificultad para tragar
- Piel seca, picor
- Daño en los músculos, debilidad muscular, dolor muscular
- Dolor de articulaciones, dolor de espalda
- Ganas de orinar frecuentes, dificultad para controlar la orina
- Fiebre, síntomas tipo gripe, dolor, sentirse mal, un constipado o gripe
- Retención de líquidos, hinchazón de piernas
- Elevación de las enzimas hepáticas
- Pérdida de peso, aumento de peso
Lesión por radiación
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- Infecciones en el cerebro (meningoencefalitis herpética) incluyendo casos fatales
- Infecciones por citomegalovirus nuevas o reactivadas
- Infecciones por el virus de la hepatitis B reactivadas
- Cancers secundarios incluyendo leucemia
- Recuento de células sanguineas reducido (pancitopenia, anemia, leucopenia)
- Puntos rojos bajo la piel
- Diabetes insipidus (síntomas que incluyen aumento de las ganas de orinar y sentirse con sed), bajos niveles de potasio en sangre
- Cambios de humor, alucinaciones
- Parálisis parcial, cambios en su sentido del olfato
- Alteraciones en la audición, infección del oído medio
- Palpitaciones (cuando puedes sentir el latido de tu corazón), sofocos
- Hinchazón de estómago, dificultat en controlar tus movimientos intestinales, hemorroides, boca seca
- Hepatitis y daño en el hígado (incluyendo fallo del hígado fatal), colestasis, aumento de la bilirrubina
- Ampollas en el cuerpo y en la boca, descamación de la piel, erupción de la piel, enrojecimiento doloroso de la piel, erupción severa con hinchazón de la piel (incluyendo palmas y plantas)
- Aumento de la sensibilidad a la luz solar, urticaria, aumento de la sudoración, cambio en el color de su piel
- Dificultad para orinar
- Sangrado vaginal, irritación vaginal, periodos menstruales ausentes o fuertes, dolor en los pechos, impotencia sexual
- Escalofríos, hinchazón de la cara, decoloración de la lengua, sed, trastorno dental
Comunicación de efectos adversos
Si experimenta efectos adversos , consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Temozolomida Accord
Mantener este medicamento fuera de la vista y del alcance de los niños, preferiblemente en un armario cerrado con llave. La ingestión accidental de las cápsulas puede ser mortal en niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja. La fecha de caducidad es el último día del mes que se indica.
Frasco
No conservar a temperatura superior a 25°C.
Conservar en el frasco original. Conservar el frasco bien cerrado para protegerlo de la humedad.
Sachet
No conservar a temperatura superior a 25ºC
Conservar el envase origininalpar protegerlo de la humedad.
Informe a su farmacéutico si observa algún cambio en el aspecto de las cápsulas.
No tire los medicamentos por los desagües ni a la basura. Pregunte a su farmacéutico donde tirar los medicamentos que ya no utiliza. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Temozolomida Accord
El principio activo es temozolomida.
Temozolomida Accord 5 mg cápsula duras: Cada cápsula contiene 5 mg de temozolomida.
Temozolomida Accord 20 mg cápsula duras: Cada cápsula contiene 20 mg de temozolomida.
Temozolomida Accord 100 mg cápsula duras: Cada cápsula contiene 100 mg de temozolomida.
Temozolomida Accord 140 mg cápsula duras: Cada cápsula contiene 140 mg de temozolomida.
Temozolomida Accord 180 mg cápsula duras: Cada cápsula contiene 180 mg de temozolomida.
Temozolomida Accord 250 mg cápsula durasCada cápsula contiene 250 mg de temozolomida.
- Los demás componentes son:
Contenido de la cápsula:
lactosa anhidra, sílice anhidra coloidal, glicolato sódico de almidón tipo A, ácido tartárico, ácido esteárico.
Envoltura de la cápsula:
Temozolomida Accord 5 mg cápsula duras: gelatina, dióxido de titanio (E 171), óxido de hierro amarillo (E 172), índigo carmín (E 132), agua.
Temozolomida Accord 20 mg cápsula duras: gelatina, dióxido de titanio (E 171), óxido de hierro amarillo (E 172), agua.
Temozolomida Accord 100 mg cápsula duras: gelatina, dióxido de titanio (E 171), y óxido de hierro rojo (E172), agua.
Temozolomida Accord 140 mg cápsula duras: gelatina, dióxido de titanio (E 171), índigo carmín (E132), agua.
Temozolomida Accord 180 mg cápsula duras: gelatina, dióxido de titanio (E 171 óxido de hierro amarillo (E172), óxido de hierro rojo (E172), agua.
Temozolomida Accord 250 mg cápsula duras: gelatina, dióxido de titanio (E 171), agua. tinta de impresión:
goma laca, propilenglicol, óxido de hierro negro (E172) e hidróxido de potasio.
Aspecto del producto y contenido del envase
Temozolomida Accord 5 mg cápsulas duras tienen un cuerpo blanco, una tapa verde y están impresas con tinta negra, TMZ en la tapa y “5” en el cuerpo de la cápsula.
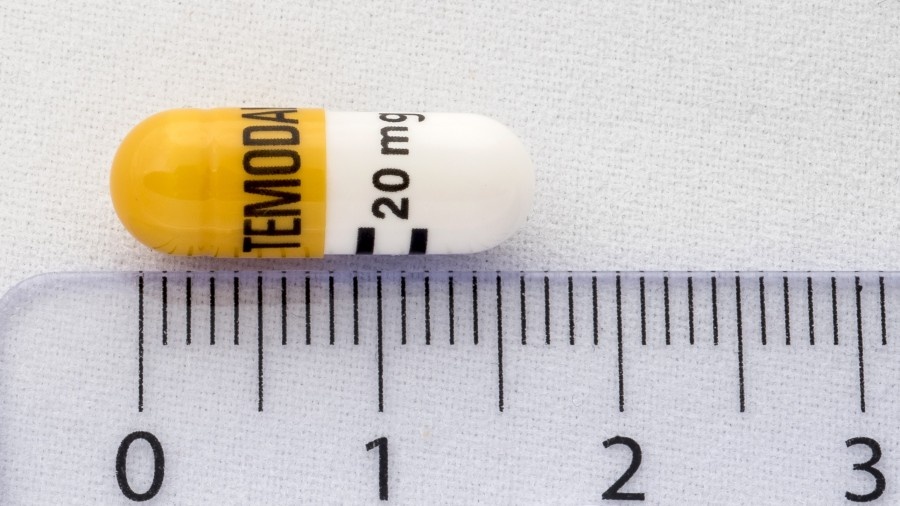
Temozolomida Accord 20 mg cápsulas duras tienen un cuerpo blanco, una tapa amarilla y están impresas con tinta negra, TMZ en la tapa y “20” en el cuerpo de la cápsula.
Temozolomida Accord 100 mg cápsulas duras tienen un cuerpo blanco, una tapa rosa y están impresas con tinta negra, TMZ en la tapa y “100” en el cuerpo de la cápsula.
Temozolomida Accord 140 mg cápsulas duras tienen un cuerpo blanco, una tapa azul y están impresas con tinta negra, TMZ en la tapa y “140” en el cuerpo de la cápsula.
Temozolomida Accord 180 mg cápsulas duras tienen un cuerpo blanco, una tapa granate y están impresas con tinta negra, TMZ en la tapa y “180” en el cuerpo de la cápsula.
Temozolomida Accord 250 mg cápsulas duras tienen un cuerpo blanco, una tapa blanca y están impresas con tinta negra, TMZ en la tapa y “250” en el cuerpo de la cápsula.
Las cápsulas duras se presentan en frascos de cristal de color ámbar que contienen 5 ó 20 cápsulas. Cada estuche contiene un frasco.
Las cápsulas duras se dispensan en sachet que contiene 1 cápsula.
Cada caja contiene 5 o 20 sachets.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación:
Titular de la autorización de comercialización
Accord Healthcare S.L.U.
World Trade Center, Moll de Barcelona, s/n,
Edifici Est 6ª planta,
08039 Barcelona, España
Fabricante
Accord Healthcare Polska Sp.z o.o.,
ul. Lutomierska 50,95-200 Pabianice, Polonia
Accord Healthcare B.V.,
Winthontlaan 200,
3526 KV Utrecht,
Países Bajos
La última revisión de este prospecto fue en XX/YYYY
Otras fuentes de información
La información detallada de este producto está disponible en la página web de la Agencia Europea del Medicamento (EMA) http://www.ema.europa.eu/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TEMOZOLOMIDA ACCORD 20 mg CAPSULAS DURAS EFGForma farmacéutica: CAPSULA, 100 mgPrincipio activo: TemozolomidaFabricante: Merck Sharp & Dohme B.V.Requiere recetaForma farmacéutica: CAPSULA, 100 mgPrincipio activo: TemozolomidaFabricante: Merck Sharp & Dohme B.V.Requiere recetaForma farmacéutica: CAPSULA, 140 mgPrincipio activo: TemozolomidaFabricante: Merck Sharp & Dohme B.V.Requiere receta
Médicos online para TEMOZOLOMIDA ACCORD 20 mg CAPSULAS DURAS EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TEMOZOLOMIDA ACCORD 20 mg CAPSULAS DURAS EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






