
TAU-KIT COMPRIMIDOS SOLUBLES


Cómo usar TAU-KIT COMPRIMIDOS SOLUBLES
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
TAU-KIT®100 mg comprimidos solubles
13C-Urea
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es TAU-KIT® y para qué se utiliza
- Qué necesita saber antes de empezar a usar TAU-KIT®
- Cómo usar TAU-KIT®
- Posibles efectos adversos
- Conservación de TAU-KIT®
- Contenido del envase e información adicional
1. Qué es TAU-KIT® y para qué se utiliza
TAU-KIT®pertenece al grupo de medicamentos denominados otros agentes diagnósticos. TAU-KIT®es un test de aliento que está indicado para determinar la presencia, en su estómago, de una bacteria denominada Helicobacter pylori, que puede ser la responsable de las molestias que usted padece.
Este medicamento es únicamente para uso diagnóstico.
2. Qué necesita saber antes de empezar a usar TAU-KIT®
No tome TAU-KIT®
Si es alérgico al principio activo o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Si padece una enfermedad genética denominada Fenilcetonuria.
Advertencias y precauciones
Consulte a su médico antes de empezar a tomar TAU-KIT®
- Un resultado positivo del test, aisladamente, no constituye indicación absoluta para un tratamiento de erradicación.
Puede estar indicado el diagnóstico con otros métodos (endoscopia), a fin de examinar la presencia de cualquier otra complicación, por ejemplo, úlcera, gastritis autoinmune o tumores malignos.
- Si usted ha sufrido una extirpación parcial de su estómago (gastrectomía) os si es menor de 5 años debe informar a su médico, ya que en estos casos no hay datos suficientes para recomendarle su uso.
- Si usted padece un tipo de gastritis denominada atrófica, el test de aliento puede tener resultados falsos positivos por lo que puede ser necesario someterle a otros estudios para confirmar la infección.
- Si usted vomita durante la prueba es necesario repetirla, como mínimo al día siguiente en ayunas.
Otros medicamentos y TAU-KIT®
Si usted está en tratamiento con antibióticos para la infección por Helicobacter pyloridebe dejar transcurrir 4 semanas sin medicación antes de la realización de la prueba de diagnóstico con TAU-KIT®, puesto que podría dar lugar a un resultado falso negativo de la misma.
Si usted está sometido a un tratamiento con un antiácido (medicamento para aliviar el ardor de estómago como omeprazol, etc) o un fármaco antiulceroso (sales de bismuto, etc) debe dejar transcurrir 2 semanas sin medicación antes de realizar el test de diagnóstico.
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar otros medicamentos.
Uso de TAU-KIT®con alimentos y bebidas
Como le indicará su médico, antes de la realización de la prueba deberá estar en ayunas durante al menos 6 horas, preferiblemente desde la noche anterior y deberá tomar 200 ml de una bebida rica en ácido cítrico (Citral pylori®) al iniciar la prueba, en el caso de los adultos, y 100 ml en el caso de niños mayores de 5 años.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, o cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
No se ha descrito ningún efecto sobre la capacidad para conducir o utilizar máquinas, a la dosis recomendada.
Información importante sobre algunos de los componentes de Citral pylori®.
La realización de la prueba del aliento con urea marcada con 13C requiere la administración previa de Ácido cítrico (Protocolo Europeo). El envase de Tau-kit incluye un sobre de Citral pylori® (4,2 g de Ácido cítrico anhidro). Cada sobre de Citral Pylori® contiene además aspartamo (E-951) y amarillo anaranjado S (E-110)
Cada sobre contiene 120 mg de aspartamo equivalente a 60 mg/100 ml.
El aspartamo contiene una fuente de fenilalanina que puede ser perjudicial en caso de padecer fenilcetonuria (FCN), una enfermedad genética rara en la que la fenilalanina se acumula debido a que el organismo no es capaz de eliminarla correctamente.
Este medicamento puede producir reacciones alérgicas porque contiene amarillo anaranjado S (E-110). Puede provocar asma, especialmente en pacientes alérgicos al ácido acetilsalicílico.
3. Cómo usar TAU-KIT®
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
TAU-KIT®es un test de aliento con 13C urea, que se utiliza para la realización de una prueba diagnóstica, la cual se debe realizar en presencia de personal cualificado, que le indicará en cada momento las instrucciones a seguir.
Para la correcta realización de la prueba, que dura aproximadamente 40 minutos, y que se debe realizar preferentemente por la mañana, es necesario que permanezca 6 horas en ayunas, sin fumar y en posición de reposo. La prueba se inicia con la ingestión del contenido del sobre Citral pylori®.
La dosis normal es:
Adultos mayores de 18 años: un comprimido (100 mg de 13C-urea) disuelto en 125 ml de agua.
Uso en niños mayores de 5 años y adolescentes:
No hay datos suficientes para recomendar su uso en pacientes menores de cinco años.
Niños mayores de 5 años: medio comprimido (50 mg de 13C-urea) disuelto en 50 ml de agua.
Forma de administración:
El medicamento se administra por vía oral.
Si mientras está realizando la prueba sufriera vómitos, se debe realizar una segunda prueba pero no antes de 24 horas.
Siga estas instrucciones a menos que su médico le haya dado otras distintas.
Si toma más TAU-KIT®del que debe
Debido a que el envase contiene un único comprimido de 100 mg de 13C-urea, no cabe esperar una sobredosificación. Ahora bien, si usted ha tomado más TAU-KIT® del que debe, consulte inmediatamente a su médico o a su farmacéutico.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o acuda a un centro médico inmediatamente o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar TAU-KIT®
Debido a que el producto está formado por un único comprimido y se utiliza en un momento puntual establecido por su médico, no cabe esperar el olvido de la toma del medicamento.
No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con TAU-KIT®
Se considera que el tratamiento consiste en la toma del único comprimido con 100 mg de 13C-urea del envase, no se prevé por tanto que exista interrupción del tratamiento.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, TAU-KIT® puede producir efectos adversos, aunque no todas las personas los sufran.
No se han descrito efectos adversos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento
5. Conservación de TAU-KIT®
Mantener este medicamento fuera de la vista y del alcance de los niños.
No requiere condiciones especiales de conservación.
No utilice este medicamento si observa algún signo de deterioro en el envase.
Caducidad
No utilice este medicamento después de la fecha de caducidad que aparece en el envase, después de la abreviatura CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el punto SIGRE de su farmacia. En caso de duda pregunte a su médico o farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de TAU-KIT®
- 1 comprimido soluble: el principio activo es: 13C Urea 100 mg
Los demás componentes del comprimido son: celulosa microcristalina, povidona K90, sílice coloidal, estearato magnésico y croscarmelosa sódica.
- 1 sobre de Citral pylori: Ácido cítrico anhidro, Aspartamo (E-951), Aroma de limón polvo y colorante amarillo anaranjado S (E-110).
Aspecto del producto y contenido del envase
TAU-KIT®se presenta en envases con un único comprimido soluble que contiene 100 mg de 13C urea y un sobre de Citral pylori®. Además incluye 2 tubos de recogida de muestra pre-dosis (BASAL), 2 tubos de recogida de muestras post-dosis (POST) y dos tubos flexibles, etiquetas adhesivas identificativas de la muestra, una de ellas se coloca en el lugar indicado en el estuche.
El formato de 50 kits se presenta como 50 kits de 4 tubos de recogida de muestras de aire en cada kit, 1 comprimido de 13C urea en cada kit y 1 sobre de Citral pylori® en cada kit.
Puede que solamente estén comercializados algunos de los tamaños de envase.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización y responsable de la fabricación:
ISOMED PHARMA, S.L.
c/París, nº4, Parque Empresarial Európolis
28232, Las Rozas Madrid
España
La última revisión de este prospecto fue enoctubre 2019
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
Esta información está destinada únicamente a profesionales del sector sanitario.
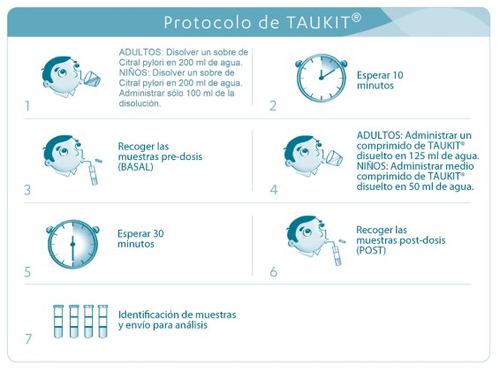
Es importante seguir estas instrucciones de manera precisa para garantizar la fiabilidad del diagnóstico.
Instrucciones específicas:
- El test debe realizarse en presencia de una persona cualificada.
- Se recomienda realizar el test estando en posición de reposo.
- La prueba se inicia con la toma de 1 sobre de Citral pylori® disuelto en 200 ml de agua en el caso de adultos y la administración de solo 100 ml de la disolución en el caso de niños mayores de 5 años. Debe anotarse la hora de ingestión.
- Diez minutos después, se lleva a cabo la recogida de muestras para la determinación del
valor basal:
- Tomar el tubo flexible y los dos tubos de recogida de muestras pre-dosis provistos de una etiqueta donde aparece la palabra “BASAL”.
- Quitar la tapa de uno de los tubos de muestra y desenvolver el tubo flexible, sin doblar, e introducirlo en el tubo de recogida de la muestra.
- Contener el aliento durante unos segundos (aprox. 30 segundos) para que la
concentración de 13CO2 en el aliento espirado sea máxima, inmediatamente, espirar
suavemente a través del tubo flexible hasta que la superficie interna del tubo de
recogida de muestra se cubra de vapor condensado.
- Continuar espirando mientras se retira el tubo de plástico e inmediatamente, cerrar el tubo de muestra con su tapa. Para cerrar el tubo se debe proceder de la siguiente forma:
enroscar hasta que el tapón no gire fácilmente y después girar con cuidado un cuarto de
vuelta más. Si el recipiente permaneciera abierto más de 30 segundos, el test podría dar
un resultado falso.
- Llenar el segundo tubo de muestra, provisto de etiqueta con la palabra “BASAL”, con la espiración y siguiendo el mismo procedimiento descrito en el punto 4.
- Preparar la solución del test: disolver el comprimido en 125 ml de agua en el caso de adultos y medio comprimido en 50 ml de agua en el caso de niños mayores de 5 años.
- Esta solución del test debe ser bebida inmediatamente por el paciente y debe anotarse la hora de ingestión.
- Treinta minutos después de la administración de la solución del test, se recogen nuevamente muestras de espiración en los tubos de recogida de muestras provistos de etiqueta con la palabra “POST” y se procede tal y como se describe en los puntos 4 y 5.
- Los tubos de recogida de muestras deben ser enviados en la caja original para su análisis a un laboratorio cualificado. Se deberá colocar en la caja la etiqueta identificativa de la muestra en el lugar señalado.
- País de registro
- Precio medio en farmacia30.46 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TAU-KIT COMPRIMIDOS SOLUBLESForma farmacéutica: COMPRIMIDO, 50 mgPrincipio activo: 13-c ureaFabricante: Laboratoires Mayoly SpindlerRequiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, DesconocidaPrincipio activo: 13-c ureaFabricante: Infai GmbhRequiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 45 mgPrincipio activo: 13-c ureaFabricante: Infai GmbhRequiere receta
Médicos online para TAU-KIT COMPRIMIDOS SOLUBLES
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TAU-KIT COMPRIMIDOS SOLUBLES, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















