
TANOLUX 50 MICROGRAMOS/ML COLIRIO EN SOLUCIÓN EN ENVASE UNIDOSIS

Cómo usar TANOLUX 50 MICROGRAMOS/ML COLIRIO EN SOLUCIÓN EN ENVASE UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
Tanolux 50 microgramos/ml colirio en solución en envase unidosis
latanoprost
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, o al médico que está tratando a su hijo, o al farmacéutico.
- Este medicamento se le ha recetado solamente a usted o a su hijo y no debe dárselo a otras personas, aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, o al médico que está tratando a su hijo o al farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Tanolux y para qué se utiliza
- Qué necesita saber antes de empezar a usar Tanolux
- Cómo usar Tanolux
- Posibles efectos adversos
- Conservación de Tanolux
- Contenido del envase e información adicional
1. Qué es Tanolux y para qué se utiliza
Latanoprost pertenece al grupo de medicamentos conocidos como análogos de las prostaglandinas. Actúa aumentando la salida natural de líquido desde el interior del ojo al torrente sanguíneo.
Latanoprost se utiliza para tratar unas enfermedades conocidas como glaucoma de ángulo abierto e hipertensión ocularen adultos. Ambas enfermedades están relacionadas con un aumento de la presión dentro del ojo, lo que puede llegar a afectar a la visión.
Latanoprost también se utiliza para tratar el aumento de la presión dentro del ojo y el glaucoma en niños y bebés de todas las edades.
2. Qué necesita saber antes de empezar a usar Tanolux
Latanoprost puede utilizarse en hombres y mujeres adultos (incluyendo ancianos) y en niños desde su nacimiento hasta los 18 años de edad. Latanoprost no ha sido investigado en niños prematuros (menos de 36 semanas de gestación).
N
- Si es alérgico (hipersensible) a latanoprost o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
A
Si considera que alguna de las siguientes situaciones le afecta a usted o a su hijo consulte con su médico, o con el médico que está tratando a su hijo, o el farmacéutico antes de usar Tanolux o antes de administrárselo a su hijo:
- Si usted o su hijo han sufrido o van a sufrir una intervención quirúrgica ocular (incluyendo una operación de cataratas).
- Si usted o su hijo padecen problemas en los ojos (tales como dolor en el ojo, irritación o inflamación, visión borrosa).
- Si usted o su hijo tienen sequedad en los ojos.
- Si usted o su hijo padecen asma grave o el asma no está bien controlado.
- Si usted o su hijo utilizan lentes de contacto. Pueden seguir utilizando Tanolux, pero han de seguir las instrucciones que se incluyen en la sección 3 para usuarios de lentes de contacto.
- Si usted ha sufrido o está sufriendo una infección vírica del ojo causada por el virus del herpes simple (VHS).
U
Tanolux puede tener interacciones con otros medicamentos. Informe a su médico, al médico que está tratando a su hijo o al farmacéutico si usted o su hijo están usando o han usado recientemente otros medicamentos (o colirios), incluso los adquiridos sin receta médica. En particular, consulte a su médico o farmacéutico si usted sabe que está tomando prostaglandinas, análogos de prostaglandinas o derivados de prostaglandinas.
E
No debería utilizar Tanoluxsi está embarazada o en período de lactancia a menos que su médico lo considere necesario. Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
C
Al utilizar Tanolux puede aparecer visión borrosa durante un periodo de tiempo breve. Si esto le sucede, no conduzcani utilice herramientas o máquinas hasta que su visión vuelva a ser nítida de nuevo.
T
Este medicamento contiene 0,2 mg/ml de cloruro de benzalconio.
El cloruro de benzalconio puede ser absorbido por lentes de contacto blandas y puede cambiar el color de las lentes de contacto. Debe quitarse las lentes de contacto antes de utilizar este medicamento y volver a ponérselas 15 minutos después.
El cloruro de benzalconio también puede producir irritación ocular, especialmente si tiene ojos secos o trastornos de la córnea (la parte frontal transparente del ojo). Si tiene una sensación anómala en los ojos, escozor o dolor en los ojos después de utilizar este medicamento, consulte a su médico.
Este medicamento contiene 6,3 mg/ml de fosfatos, lo que equivale a 0,2 mg/gota.
Si sufre un daño grave en la parte frontal transparente del ojo (la córnea), los fosfatos pueden provocar, en casos muy raros, áreas nubladas en la córnea debido a los depósitos de calcio producidos durante el tratamiento.
3. Cómo usar Tanolux
Siga exactamente las instrucciones de administración de Tanolux indicadas por su médico, o por el médico que trata a su hijo. Consulte a su médico, o al médico que trata a su hijo, o al farmacéutico si tiene dudas.
La dosis recomendada para adultos (incluyendo ancianos) y niños, es de una gota en el ojo o en los ojos afectados una vez al día. Es preferible que se administre por la noche.
No utilice Tanolux más de una vez al día; la eficacia del tratamiento puede disminuir si se administra con mayor frecuencia.
Utilice Tanolux tal y como su médico o el médico que trata a su hijo le ha indicado hasta que le diga que lo suspenda.
U
Si usted o su hijo utilizan lentes de contacto, debe quitárselas antes de utilizar Tanolux. Después de la aplicación de Tanolux, debe esperar 15 minutos antes de volver a ponerse las lentes de contacto.
I
Lávese las manos antes de utilizarlo. Asegúrese de que el envase unidosis está intacto antes de utilizar este medicamento. La solución debe utilizarse inmediatamente después de abrir el envase. Para evitar contaminación, no deje que la punta del envase unidosis toque el ojo ni ninguna otra superficie.
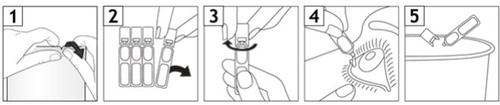
1,2, 3. Coja un envase unidosis de la bolsa y manténgalo en posición vertical (con el tapón hacia arriba) y gire el tapón hasta que se desprenda.
- Tire suavemente del párpado inferior hacia abajo para formar una bolsa. Invierta el envase unidosis y apriete hasta que caiga una gota en el ojo o los ojos afectados.
- Deseche el envase unidosis después de su uso, aunque quede solución en su interior.
S
Espere al menos 5 minutos entre la aplicación de Tanolux y la administración de otros colirios.
S
Si se ha aplicado más gotas en el ojo de las que debía, puede sentir una ligera irritación en el ojo y también puede que los ojos se pongan rojos y que lloren; esta situación debería desaparecer, pero si le preocupa, contacte con su médico o con el médico que trata a su hijo.
En caso de una ingestión accidental suya o de su hijo de Tanolux, consulte con su médico o farmacéutico lo antes posible, o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20.
S
Continúe con la administración de la siguiente dosis de la forma habitual. No debe aplicarse una gota adicional en el ojo para compensar la dosis olvidada. Si tiene dudas consulte con su médico o farmacéutico.
S
Si desea dejar de utilizar Tanolux, consulte con su médico o con el médico que trata a su hijo.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los siguientes son efectos adversos conocidos cuando se usa Tanolux:
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 pacientes):
- Cambio gradual en el color de los ojos por el incremento de la cantidad de pigmento de color marrón de la parte coloreada del ojo conocida como iris. Si tiene ojos de color mixto (azul-marrón, gris-marrón, amarillo-marrón o verde-marrón) es más probable que sufra este cambio que si sus ojos son de un solo color (azul, gris, verde o marrón). El cambio en el color de los ojos tarda años en desarrollarse, aunque puede apreciarse normalmente a los 8 meses de tratamiento. El cambio de color puede ser permanente y puede ser más llamativo si Tanolux se utiliza únicamente en un ojo. El cambio en el color del ojo no parece estar asociado a la aparición de ningún problema. El cambio en el color del ojo no progresa una vez que se ha suspendido el tratamiento con Tanolux.
- Enrojecimiento del ojo.
- Irritación ocular (sensación de escozor, sensación de arenilla en el ojo, picor, dolor y sensación de cuerpo extraño en el ojo). Si experimenta irritación ocular grave que le provoca que los ojos le lloren excesivamente o que le haga considerar interrumpir el tratamiento, consulte con su médico, farmacéutico o enfermera a la mayor brevedad posible (en una semana). Puede requerir que se revise su tratamiento para garantizar que está recibiendo el tratamiento adecuado para su condición.
- Cambio gradual en las pestañas del ojo tratado y del vello fino que hay alrededor del ojo tratado, observado en la mayoría de los pacientes de origen japonés. Estos cambios incluyen un aumento del color (oscurecimiento), alargamiento, engrosamiento y aumento del número de pestañas.
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 pacientes):
- Irritación o erosión en la superficie del ojo, inflamación del párpado (blefaritis), dolor en el ojo y sensibilidad a la luz (fotofobia), conjuntivitis.
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 pacientes):
- Hinchazón de los párpados, ojo seco, inflamación o irritación de la superficie del ojo (queratitis), visión borrosa, inflamación de la parte coloreada del ojo (uveítis), hinchazón de la retina (edema macular).
- Erupción de la piel.
- Dolor de pecho (angina), sentir el ritmo cardiaco (palpitaciones).
- Asma, dificultad en la respiración (disnea).
- Dolor de pecho.
- Dolor de cabeza, mareo.
- Dolor muscular, dolor articular.
- Náuseas, vómitos.
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 pacientes):
- Inflamación del iris (iritis), síntomas de hinchazón o lesión/daño en la superficie del ojo, hinchazón alrededor del ojo (edema periorbitario), pestañas desviadas o hilera adicional de pestañas, cicatrización de la superficie del ojo, acumulación de líquido en la parte coloreada del ojo (quiste del iris), sensibilidad a la luz (fotofobia).
- Reacciones en la piel de los párpados, oscurecimiento de la piel de los párpados.
- Empeoramiento del asma.
- Picor intenso en la piel.
- Desarrollo de una infección vírica del ojo causada por el virus del herpes simple (VHS).
Efectos adversos muy raros(pueden afectar hasta 1 de cada 10.000 pacientes):
- Agravamiento de la angina en pacientes que también tienen problemas cardiacos, apariencia de ojos hundidos (mayor profundidad del surco del párpado).
Los efectos adversos observados en niños a una frecuencia mayor que en adultos son moqueo y picor de nariz y fiebre.
En casos muy raros, algunos pacientes con un daño grave en la parte frontal transparente del ojo (córnea) han desarrollado áreas nubladas en la córnea debido a los depósitos de calcio producidos durante el tratamiento.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
C:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Tanolux
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el estuche después de EXP. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere ninguna temperatura especial de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
El principio activo es latanoprost 50 microgramos/ml.
Los demás componentes son: cloruro de benzalconio, cloruro de sodio, dihidrogenofosfato de sodio monohidrato (E339i), hidrogenofosfato de sodio anhidro (E339ii) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Tanolux colirio es un líquido transparente e incoloro que se suministra en envases unidosis de plástico, cada uno de los cuales contiene 0,4 ml de solución.
La caja contiene 3, 6, 9 bolsas de aluminio, cada una con 10 envases unidosis, con un total de 30, 60, 90 envases unidosis en la caja respectivamente.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Laboratorios Salvat, S.A.
Gall, 30-36 - 08950
Esplugues de Llobregat (Barcelona)
España
Responsable de la fabricación
Pharmaloop S.L.
C/Bolivia, 15 – Polig Industrial Azque
28806 Alcalá de Henares,
Madrid – España
Laboratorios Salvat, S.A.
Gall, 30-36 - 08950
Esplugues de Llobregat (Barcelona)
España
Fecha de la última revisión de este prospecto: Enero 2023
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con siguientes nombres:
España Tanolux 50 microgramos/ml colirio en solución en envase unidosis
Portugal Tanolux 0,02 mg/ 0,4 ml colírio, solução em recipiente unidose
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia15.61 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TANOLUX 50 MICROGRAMOS/ML COLIRIO EN SOLUCIÓN EN ENVASE UNIDOSISForma farmacéutica: COLIRIO, 50 microgramos/mlPrincipio activo: LatanoprostFabricante: Santen OyRequiere recetaForma farmacéutica: COLIRIO, 50 µg/mlPrincipio activo: LatanoprostFabricante: Esteve Pharmaceuticals S.A.Requiere recetaForma farmacéutica: COLIRIO, 50 MG/MLPrincipio activo: LatanoprostFabricante: Aurovitas Spain, S.A.U.Requiere receta
Médicos online para TANOLUX 50 MICROGRAMOS/ML COLIRIO EN SOLUCIÓN EN ENVASE UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TANOLUX 50 MICROGRAMOS/ML COLIRIO EN SOLUCIÓN EN ENVASE UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















