
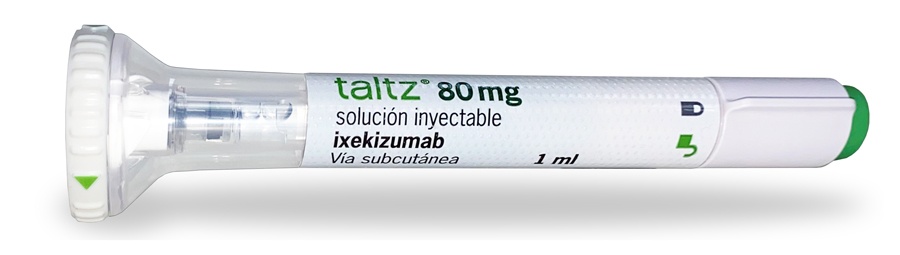
TALTZ 80 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar TALTZ 80 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Taltz 80mg solución inyectable en jeringa precargada
ixekizumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Taltz y para qué se utiliza
- Qué necesita saber antes de empezar a usar Taltz
- Cómo usar Taltz
- Posibles efectos adversos
- Conservación de Taltz
- Contenido del envase e información adicional
1. Qué es Taltz y para qué se utiliza
Taltz contiene el principio activo ixekizumab.
Taltz se utiliza para el tratamiento de las enfermedades inflamatorias que se describen a continuación:
- Psoriasis en placas en adultos
- Psoriasis en placas en niños a partir de 6 años y con un peso corporal de al menos 25 kg y en adolescentes
- Artritis psoriásica en adultos
- Espondilitis anquilosante en adultos
- Espondiloartritis axial no radiográfica en adultos
Ixekizumab pertenece a un grupo de medicamentos llamados inhibidores de interleuquinas (IL). Este medicamento actúa bloqueando la actividad de una proteína denominada IL-17A, que promueve la psoriasis y enfermedades inflamatorias de las articulaciones y de la columna vertebral.
Psoriasis en placas
Taltz se usa para el tratamiento de un trastorno de la piel conocido como “psoriasis en placas” en adultos y en niños desde 6 años con un peso corporal de al menos 25 kg y en adolescentes con enfermedad de moderada a grave. Taltz reduce los signos y síntomas de la enfermedad.
El uso de Taltz le beneficiará porque produce mejorías en el aspecto de las lesiones de la piel y la disminución de síntomas tales como la descamación, el picor y el dolor.
Artritis psoriásica
Taltz se usa para el tratamiento de un trastorno conocido como “artritis psoriásica” en adultos, una enfermedad inflamatoria de las articulaciones, a menudo acompañada de psoriasis. Si padece artritis psoríasica, recibirá primero otros medicamentos. Si no responde suficientemente bien o no tolera estos medicamentos, recibirá Taltz para reducir signos y síntomas de la enfermedad. Taltz se puede utilizar solo o con otro medicamento llamado metotrexato.
Utilizar Taltz le beneficiará reduciendo los signos y síntomas de la enfermedad, mejorando la función física (habilidad para realizar las actividades diarias normales), y ralentizando el daño en las articulaciones.
Espondiloartritis axial
Taltz se utiliza para tratar a adultos con una enfermedad inflamatoria que afecta principalmente a la columna vertebral y que provoca la inflamación de las articulaciones de la columna, llamada espondiloartritis axial. Si la afección es visible mediante rayos X, se denomina "espondilitis anquilosante o espondiloartritis axial radiográfica"; si se produce en pacientes sin signos visibles en los rayos X, se denomina "espondiloartritis axial no radiográfica". Si tiene espondiloartritis axial, primero se le administrarán otros medicamentos. Si no responde lo suficientemente bien a estos medicamentos, se le administrará Taltz para reducir los signos y síntomas de la enfermedad, disminuir la inflamación y mejorar su función física.
2. Qué necesita saber antes de empezar a usar Taltz
No use Taltz
- si es alérgico a ixekizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Si cree que puede ser alérgico, consulte con su médico antes de usar Taltz.
- si tiene alguna infección que su médico considere importante (por ejemplo, tuberculosis activa).
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Taltz:
- si actualmente tiene una infección o si padece infecciones repetidas o prolongadas.
- si padece una enfermedad inflamatoria que afecta al intestino llamada enfermedad de Crohn.
- si padece una inflamación del intestino grueso llamada colitis ulcerosa.
- si recibe algún otro tratamiento para la psoriasis (como inmunosupresores o fototerapia con luz ultravioleta) o para la artritis psoriásica.
Enfermedad inflamatoria intestinal (enfermedad de Crohn o colitis ulcerosa)
Deje de utilizar Taltz e informe a su médico o busque atención médica inmediatamente si nota calambres abdominales y dolores, diarrea, pérdida de peso o sangre en las heces (cualquier signo de problemas intestinales).
Si no está seguro de estar en alguna de las situaciones anteriores, consulte a su médico o enfermero antes de utilizar Taltz.
Vigile la aparición de infecciones y reacciones alérgicas
Taltz puede ocasionar potencialmente efectos adversos graves, incluyendo infecciones y reacciones alérgicas. Debe vigilar la aparición de signos de estas enfermedades mientras use Taltz.
Interrumpa el tratamiento con Taltz y avise a su médico o busque asistencia médica inmediatamente si nota cualquier signo de infección grave o una reacción alérgica. Estos signos se incluyen en la sección 4 “Efectos adversos graves”.
Niños y adolescentes
No utilice este medicamento para el tratamiento de psoriasis en placas en niños menores de 6 años porque no ha sido estudiado en este grupo de edad.
No utilice este medicamento para el tratamiento de artritis psoriásica en niños y adolescentes menores de 18 años porque no se ha estudiado el medicamento en este grupo de edad.
Otros medicamentos y Taltz
Informe a su médico, farmacéutico o enfermero:
- si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
- si ha sido vacunado recientemente o va a ser vacunado. No le deben administrar algunos tipos de vacunas mientras use Taltz.
Embarazo y lactancia
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Es preferible que evite el uso de Taltz durante el embarazo. Se desconoce los efectos de este medicamento en mujeres embarazadas. Si es una mujer en edad fértil, se aconseja que evite quedarse embarazada y debe utilizar un anticonceptivo adecuado mientras use Taltz y durante al menos 10 semanas después de la última dosis de Taltz.
Si está dando el pecho o tiene previsto dar el pecho, consulte a su médico antes de utilizar este medicamento. Usted y su médico deben decidir si puede dar el pecho o va a utilizar Taltz. No debe hacer las dos cosas a la vez.
Conducción y uso de máquinas
Es poco probable que Taltz influya sobre su capacidad para conducir o utilizar máquinas.
Taltz contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por 80 mg de dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Taltz
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o enfermero. En caso de duda, consulte de nuevo a su médico, enfermero o farmacéutico.
Taltz se administra por una inyección debajo de la piel (inyección subcutánea). Usted y su médico o enfermero deberían decidir si debe ser usted el que se inyecte Taltz.
Para el uso en niños con un peso corporal de 25-50 kg la dosis de 40 mg de ixekizumab se debe preparar y administrar por un profesional sanitario cualificado.
Es importante que usted no intente inyectarse el medicamento hasta que haya sido entrenado en cómo hacerlo por su médico o enfermero. Un cuidador también puede ser el que le administre su inyección de Taltz si ha sido entrenado adecuadamente.
Utilice algún método de recuerdo como notas en un calendario o una agenda, que le ayuden a recordar cuando tiene su próxima dosis y así evitar olvidos o dosis repetidas.
Taltz es un tratamiento de larga duración. Su médico o enfermero controlarán periódicamente el estado de su enfermedad para comprobar si el tratamiento tiene el efecto deseado.
Cada jeringa contiene una dosis de Taltz (80 mg). Cada jeringa proporciona solo una dosis. La jeringa no se debe agitar.
Lea atentamente las “Instrucciones de uso” de la jeringa antes de usar Taltz.
Cuánto Taltz se debe administrar y durante cuánto tiempo
Su médico le explicará cuánto Taltz necesita usted y durante cuánto tiempo.
Psoriasis en placas en adultos
- La primera dosis es de 160 mg (2 jeringas de 80 mg cada una) por inyección subcutánea. Esta dosis puede que le sea administrada por su médico o enfermero.
- Tras la primera dosis, usará una dosis de 80 mg (1 jeringa) a la semana 2, 4, 6, 8, 10 y 12. Desde la semana 12 en adelante usará una dosis de 80 mg (1 jeringa) cada 4 semanas.
Psoriasis en placas en niños (6 años o más y un mínimo de 25 kg de peso corporal) y en adolescentes.
La dosis recomendada administrada por inyección subcutánea en niños se basa en las siguientes categorías de peso:
Peso corporal del niño | Dosis recomendada de inicio(semana0) | Dosis recomendada cada 4semanas(Q4W)posteriormente |
Más de 50 kg | 160 mg (2 jeringas) | 80 mg (1 jeringa) |
25 a 50 kg | 80 mg (1 jeringa) | 40 mg (requiere preparación de dosis) |
Preparación de40mgdeixekizumaben niños
Las dosis de ixekizumab 40 mg se deben preparar y administrar por un profesional sanitario cualificado.
No se recomienda el uso de Taltz en niños con un peso corporal inferior a 25 kg.
Artritis psoriásica
Para pacientes con artritis psoriásica que además padecen psoriasis en placas de moderada a grave:
- La primera dosis es 160 mg (2 jeringas de 80 mg cada una) por inyección subcutánea. Esta dosis puede que le sea administrada por su médico o enfermero.
- Tras la primera dosis, usará una dosis de 80 mg (1 jeringa) a la semana 2, 4, 6, 8, 10 y 12. Desde la semana 12 en adelante usará una dosis de 80 mg (1 jeringa) cada 4 semanas.
Para el resto de pacientes con artritis psoriásica
- La primera dosis es 160 mg (2 jeringas de 80 mg cada una) por inyección subcutánea. Esta dosis puede que le sea administrada por su médico o enfermero.
- Después de la primera dosis usará una dosis de 80 mg (1 jeringa) cada 4 semanas.
Espondiloartritis axial
La dosis recomendada es 160 mg (2 jeringas de 80 mg cada una) por inyección subcutánea en la semana 0, seguida de 80 mg (1 jeringa) cada 4 semanas.
Si usa más Taltz del que debe
Si ha recibido más Taltz del que debe o la dosis ha sido administrada antes de lo que le habían indicado, informe a su médico.
Si olvidó usar Taltz
Si ha olvidado inyectarse una dosis de Taltz, hable con su médico.
Si interrumpe el tratamiento con Taltz
No debe interrumpir el uso de Taltz sin hablar antes con su médico. Si deja el tratamiento, los síntomas de la psoriasis o de la artritis psoriásica pueden reaparecer.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Interrumpa el tratamiento con Taltz y consulte a su médico o busque asistencia médica inmediata si nota alguno de los siguientes efectos adversos. Su médico decidirá si debe y cuándo puede reiniciar el tratamiento:
Posible infección grave(puede afectar hasta 1 de cada 100 personas) – los signos pueden incluir:
- fiebre, síntomas gripales, sudores nocturnos
- sensación de cansancio o dificultad para respirar, tos persistente
- piel dolorosa, enrojecida o caliente, o erupción en la piel dolorosa con ampollas
Reacción alérgica grave(puede afectar hasta 1 de cada 1000 personas) – los signos pueden incluir:
- dificultad para respirar o tragar
- tensión arterial baja, que puede ocasionar mareo o ligero aturdimiento
- hinchazón de la cara, labios, lengua o garganta
- picor intenso de la piel acompañado de erupción o ronchas
Otros efectos adversos comunicados:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- infecciones de las vías respiratorias altas con síntomas como dolor de garganta y congestión nasal.
- reacciones en el lugar de la inyección (p.ej. piel enrojecida, dolor).
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- náuseas.
- infección por hongos como por ejemplo el pie de atleta.
- dolor en la parte posterior de la garganta.
- úlceras bucales, en la piel y en las membranas mucosas (herpes simple, mucocutáneo).
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- lesiones blanquecinas en la boca (candidiasis oral).
- gripe.
- congestión nasal.
- infección bacteriana de la piel.
- urticaria.
- ojos con lagrimeo, picor, enrojecimiento e hinchazón (conjuntivitis).
- signos de niveles bajos de glóbulos blancos en la sangre, como fiebre, dolor de garganta o úlceras en la boca debidas a infección (neutropenia).
- número bajo de plaquetas en la sangre (trombocitopenia).
- eccema.
- erupción.
- inflamación rápida de los tejidos del cuello, cara, boca o garganta (angioedema).
- calambres abdominales y dolor, diarrea, pérdida de peso o sangre en las heces (signos de problemas intestinales).
Raras(pueden afectar hasta 1 de cada 1000 personas)
- infección fúngica del esófago (candidiasis esofágica)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Taltz
Mantener este medicamento fuera de la vista y del alcance de los niños.
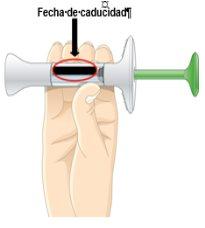
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta de la jeringa y en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (2 ºC a 8 ºC). No congelar. No empujar hacia la parte trasera del frigorífico.
Conservar en el envase original para protegerlo de la luz.
Taltz se puede dejar fuera de la nevera hasta 5 días a una temperatura no superior a 30 ºC.
No utilice este medicamento si observa que la jeringa está deteriorada o el medicamento parece turbio, es claramente de color marrón o contiene partículas en su interior.
Este medicamento es para un solo uso.
Los medicamentos no se deben tirar por los desagües. Pregunte a su médico, farmacéutico o enfermero cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Taltz
- El principio activo es ixekizumab.
Cada jeringa precargada contiene 80 mg de ixekizumab en 1 ml de solución.
- Los demás componentes son sacarosa, polisorbato 80, agua para preparaciones inyectables. Además, es posible que se haya añadido hidróxido de sodio para ajustar el pH.
Aspecto del producto y contenido del envase
Taltz es una solución en una jeringa de vidrio transparente. Su color puede variar desde transparente a ligeramente amarillo.
Envases de 1, 2, 3 jeringas precargadas. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Eli Lilly and Company (Ireland) Limited, Dunderrow, Kinsale, Co. Cork, Irlanda.
Responsable de la fabricación
Eli Lilly Italia S.p.A.,Via Gramsci 731/733, 50019, Sesto Fiorentino (FI), Italia.
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Belgique/België/Belgien Eli Lilly Benelux S.A./N.V. Tél/Tel: + 32-(0)2 548 84 84 | Lietuva Eli Lilly Lietuva Tel. +370 (5) 2649600 |
???????? ?? "??? ???? ?????????" ?.?. - ???????? ???. + 359 2 491 41 40 | Luxembourg/Luxemburg Eli Lilly Benelux S.A./N.V. Tél/Tel: + 32-(0)2 548 84 84 |
Ceská republika ELI LILLY CR, s.r.o. Tel: + 420 234 664 111 | Magyarország Lilly Hungária Kft. Tel: + 36 1 328 5100 |
Danmark Eli Lilly Danmark A/S Tlf: +45 45 26 60 00 | Malta Charles de Giorgio Ltd. Tel: + 356 25600 500 |
Deutschland Lilly Deutschland GmbH Tel. + 49-(0) 6172 273 2222 | Nederland Eli Lilly Nederland B.V. Tel: + 31-(0) 30 60 25 800 |
Eesti Eli Lilly Nederland B.V. Tel: +372 6 817 280 | Norge Eli Lilly Norge A.S. Tlf: + 47 22 88 18 00 |
Ελλ?δα ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε. Τηλ: +30 210 629 4600 | Österreich Eli Lilly Ges.m.b.H. Tel: + 43-(0) 1 711 780 |
España Lilly S.A. Tel: + 34-91 663 50 00 | Polska Eli Lilly Polska Sp. z o.o. Tel: +48 22 440 33 00 |
France Lilly France Tél: +33-(0) 1 55 49 34 34 | Portugal Lilly Portugal Produtos Farmacêuticos, Lda Tel: + 351-21-4126600 |
Hrvatska Eli Lilly Hrvatska d.o.o. Tel: +385 1 2350 999 | România Eli Lilly România S.R.L. Tel: + 40 21 4023000 |
Ireland Eli Lilly and Company (Ireland) Limited Tel: + 353-(0) 1 661 4377 | Slovenija Eli Lilly farmacevtska družba, d.o.o. Tel: +386 (0)1 580 00 10 |
Ísland Icepharma hf. Sími + 354 540 8000 | Slovenská republika Eli Lilly Slovakia s.r.o. Tel: + 421 220 663 111 |
Italia Eli Lilly Italia S.p.A. Tel: + 39- 055 42571 | Suomi/Finland Oy Eli Lilly Finland Ab Puh/Tel: + 358-(0) 9 85 45 250 |
Κ?προς Phadisco Ltd Τηλ: +357 22 715000 | Sverige Eli Lilly Sweden AB Tel: + 46-(0) 8 7378800 |
Latvija Eli Lilly (Suisse) S.A Parstavnieciba Latvija Tel: +371 67364000 | United Kingdom(Northern Ireland) Eli Lilly and Company (Ireland) Limited Tel: + 353-(0) 1 661 4377 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/, y en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
------------------------------------------------------------------------------------------------------------------------
La siguiente información está destinada únicamente a los profesionales médicos o de la salud:
Preparación de 40 mg de ixekizumab para niños de 25 a 50 kg de peso corporal
Las dosis de ixekizumab de 40 mg se deben preparar y administrar por un profesional sanitario cualificado. Utilice solo la jeringa precargada Taltz 80 mg solución inyectable cuando prepare las dosis pediátricas de 40 mg prescritas.
- Expulse todo el contenido de la jeringa precargada en un frasco de vidrio estéril y transparente. NO agite ni gire el vial.
- Utilice una jeringa desechable de 0,5 ml o 1 ml y una aguja estéril para retirar la dosis prescrita (0,5 ml para 40 mg) del vial.
- Cambie la aguja y utilice una aguja estéril de calibre 27 para inyectar al paciente. Deseche cualquier resto de ixekizumab no utilizado que quede en el vial.
La preparación de ixekizumab debe administrarse dentro de las siguientes 4 horas a la perforación del vial estéril a temperatura ambiente.
Instrucciones de uso Taltz 80mg solución inyectable en jeringa precargada ixekizumab |
|
Antes de usar su jeringa precargada: Puntos importantes que tiene que saber |
|
|
|
|
INSTRUCCIONES DE USO Antes de usar Taltz en jeringa precargada, lea y siga cuidadosamente todos los pasos de las instrucciones. |
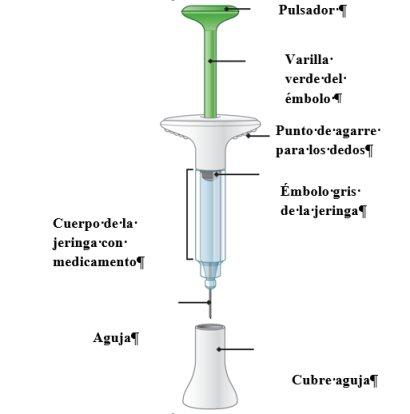
Guía de los componentes de la jeringa | ||
| ||
1 | PREPARE LA INYECCIÓN | |
1a | Saque la jeringa de la nevera.Deje el cubre aguja en la jeringa hasta que esté preparado para inyectarla. Espere 30minutospara que la jeringa se ponga a temperatura ambiente antes de usarla. NOutilice ninguna fuente de calor para aumentar la temperatura del medicamento, como por ejemplo: microondas, agua caliente o ponerlo directamente al sol. |
|
1b | Reúna los elementos necesarios para la inyección:
| |
1c |
| Inspeccione la jeringa precargada por si tiene algún deterioro que sea visible.Deje el cubre aguja en la jeringa hasta que esté preparado para inyectarla. Compruebe la etiqueta. Asegúrese de que el nombre de Taltz aparece en la etiqueta. El medicamento dentro debe ser transparente. El color puede variar de transparente a ligeramente amarillo. NO USEla jeringa y deséchela como se le indica más adelante en cualquiera de las siguientes circunstancias:
|
1d | Lávese las manos antes de inyectarse el medicamento. | |
1e |
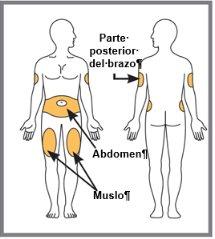
| Elija su lugar de inyección. Se puede inyectar en el abdomen (zona de la tripa), en el muslo o en la parte posterior del brazo. Para la inyección en el brazo, puede que necesite ayuda de otra persona. NOinyecte en zonas donde la piel esté dolorida, magullada, roja o dura o donde tenga cicatrices o estrías. NOinyecte en la zona comprendida en los 2,5 cm alrededor del ombligo. Alterne el lugar de inyección. NOinyecte en el mismo punto siempre. Por ejemplo, si su última inyección fue en el muslo izquierdo, su siguiente inyección debería ser en el muslo derecho, en el abdomen o la parte posterior de cualquiera de los brazos. |
1f | Prepare su piel. Limpie la piel con una toallita con alcohol. Deje que el sitio donde se va a inyectar se seque de forma natural antes de que inyecte el medicamento. | |
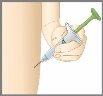
2 | INYECTE | |
2a |
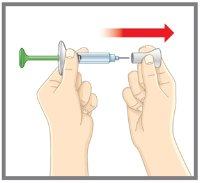
| Retire y deseche el cubre aguja. NOvuelva a poner el cubre aguja- podría dañar la aguja o hacerse daño accidentalmente. NOtoque la aguja. |
2b |
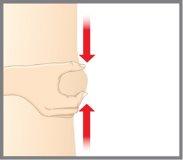
| Pellizque con suavidad y mantenga un pliegue de piel en el lugar donde vaya a realizar la inyección. |
2c |
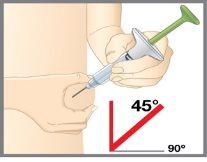
| Inserte la aguja con un ángulo de 45grados.Después suavemente deje de sujetar su piel. Asegúrese de que la aguja se mantiene en su sitio. |
| ||
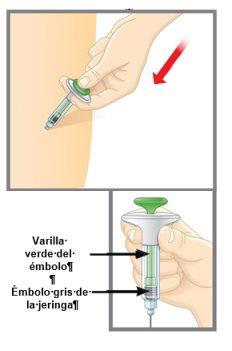
2d |
| Empuje el émbolo. Empuje lentamente el émbolo hasta el fondo hasta que se inyecte todo el medicamento. El émbolo gris de la jeringa debe avanzar hasta el final de la jeringa. Retire suavemente la aguja de su piel. Presione con un algodón o gasa sobre el lugar de la inyección. NOfrote el lugar de la inyección, ya que puede provocar moratones. Puede que sangre ligeramente. Esto es normal. Cuando la inyección ha terminado, debe ver la varilla verde del émbolo a través del cuerpo de la jeringa. |
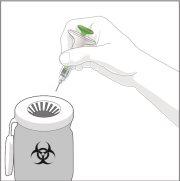
3 | TERMINE | |
| Tire la jeringa precargada. NOvuelva a poner la tapa de la aguja. Tire la jeringa a un contenedor de objetos punzantes o como le haya indicado su médico, farmacéutico o enfermero. | |
Cuando elimine la jeringa y el contenedor de objetos punzantes: | ||
| ||
Recomendaciones de seguridad | ||
| ||
Preguntas frecuentes | ||
P. | ¿Qué pasa si veo burbujas de aire en mijeringa? | |
R. | Es normal que algunas veces haya burbujas de aire en la jeringa. Taltz se inyecta bajo la piel (inyección subcutánea). En este tipo de inyección las burbujas de aire no son un problema. No le perjudicarán ni afectarán a su dosis. | |
P. | ¿Qué pasa si hay una gota de líquido en la punta de la aguja cuando retiro el cubre agujas? | |
R. | Una gota de líquido en la punta de la aguja no es extraño. No le perjudicará ni afectará a su dosis. | |
P. | ¿Qué hago si no puedo empujar el émbolo? | |
R. | Si el émbolo está atascado o deteriorado: | |
| ||
| ||
P. | ¿Cómo sé cuándo ha terminado la inyección? | |
R. | Su inyección ha terminado cuando: | |
| ||
| ||
P. | ¿Qué pasa si la jeringa se deja a temperatura ambiente durante más de 30minutos? | |
R. | Si es necesario, la jeringa se puede dejar fuera de la nevera a una temperatura no superior a 30 °C durante un máximo de 5 días si se protege de la luz solar directa. Taltz se debe desechar si no se usa en el plazo de 5 días a temperatura ambiente. | |
Para conocer más sobre su medicamento, lea las Instrucciones de uso completas y el prospecto de Taltz dentro de este envase. | ||
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TALTZ 80 mg SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 80 mgPrincipio activo: IxekizumabFabricante: Eli Lilly And Co (Ireland) LimitedRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 130 mgPrincipio activo: UstekinumabFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE, 45 mgPrincipio activo: UstekinumabFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para TALTZ 80 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TALTZ 80 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






 3a
3a
