
SUPREFACT DEPOT 9,45 mg IMPLANTE

Cómo usar SUPREFACT DEPOT 9,45 mg IMPLANTE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Suprefact Depot 9,45 mg implante
Buserelina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Suprefact Depot y para qué se utiliza
- Qué necesita saber antes de empezar a usar Suprefact Depot
- Cómo usar Suprefact Depot
- Posibles efectos adversos
- Conservación de Suprefact Depot
- Contenido del envase e información adicional
1. Qué es Suprefact Depot y para qué se utiliza
Qué es Suprefact Depot
Suprefact Depot contiene un principio activo denominado buserelina. La buserelina es similar a una hormona que se produce de forma natural en el cerebro. Pertenece a un grupo de medicamentos denominado “análogos de hormonas liberadoras de gonadotropinas” (análogos LHRH).
Cómo actúa Suprefact Depot
Actúa disminuyendo la cantidad de hormonas que estimulan el crecimiento del tumor de próstata. La próstata es una glándula que se encuentra debajo de la vejiga en hombres.
Para qué se utiliza Suprefact Depot
Suprefact Depot se utiliza para el tratamiento del cáncer de próstata avanzado.
2. Qué necesita saber antes de empezar a usar Suprefact Depot
No use Suprefact Depot:
- si es alérgico a la buserelina, a otros análogos LHRH (por ejemplo leuprolida, goserelina, triptorelina) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Los síntomas de una reacción alérgica podrían ser: erupción en la piel, problemas para tragar o respirar, hinchazón en los labios, cara, garganta o lengua.
No tome este medicamento si le ocurre algo de lo anteriormente mencionado. Si no está seguro, antes de empezar el tratamiento con Suprefact Depot pregunte a su médico o farmacéutico.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Suprefact Depot si:
- le han extirpado los testículos
- padece cáncer y éste está extendido (cáncer metastático). Al principio, es importante para usted usar otro medicamento para disminuir los niveles de ciertas hormonas. Sin embargo, esto puede causar dolor tumoral; si esto sucediera pregunte a su médico o farmacéutico
- tiene dificultades para orinar
- tiene factores de riesgo para enfermedad cardiovascular o diabetes
- padece alguna afección del corazón o los vasos sanguíneos o está siendo tratado para ello, incluyendo medicamentos para controlar el ritmo cardiaco (arritmias). El riesgo de problemas del ritmo cardiaco puede aumentar cuando se utiliza Suprefact Depot
- padece diabetes. Controle de forma habitual sus niveles de azúcar en sangre. Esto se debe a que Suprefact Depot afecta al metabolismo y por lo tanto a sus niveles de azúcar en sangre
- tiene la tensión arterial alta. Su médico o enfermera deberá controlar de forma habitual la tensión arterial. Esto se debe a que Suprefact Depot afecta a la tensión arterial
- ha sufrido alguna vez una depresión. Deberá controlar detenidamente su estado mental porque existe riesgo de que la depresión pudiera aparecer de nuevo o empeorar
- ha disminuido su número de glóbulos rojos o siente un aumento del cansancio (anemia).
Si no está seguro de si algo de lo anteriormente mencionado le afecta, consulte a su médico o farmacéutico antes de usar Suprefact Depot.
Su médico controlará la densidad de sus huesos y podría prescribirle el tratamiento apropiado. Esto es debido a que el uso de los análogos LHRH puede causar una disminución en la densidad de los huesos, osteoporosis (debilitamiento de los huesos) y un aumento del riesgo de fracturas de hueso, especialmente si tiene factores de riesgo tales como alcoholismo crónico, tabaquismo, o si en su familia ha habido más personas con osteoporosis, o si lleva mucho tiempo en tratamiento con medicamentos anticonvulsivantes o corticosteroides.
Ha habido notificaciones de depresión en pacientes que usan Suprefact Depot, que podrían ser graves. Si está usando Suprefact Depot y desarrolla un estado de ánimo depresivo, informe a su médico.
Otros medicamentos Suprefact Depot
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento. Esto incluye los comprados sin receta médica y las hierbas medicinales. Esto se debe a que Suprefact Depot puede afectar a la forma en que estos medicamentos actúan. Otros medicamentos también pueden afectar la forma en que Suprefact Depot actúa.
En particular, consulte a su médico:
- si está tomando medicamentos para la diabetes. Esto se debe a que Suprefact Depot puede afectar la forma en que estos medicamentos actúan, lo cual puede llevar a un empeoramiento de la diabetes.
- si está utilizando medicamentos usados para tratar problemas del ritmo del corazón (por ejemplo: quinidina, procainamida, amiodarona y sotalol). Esto es porque Suprefact Depot puede interferir con estos medicamentos.
- si está utilizando otros medicamentos (por ejemplo: metadona (utilizado para el alivio del dolor y para la desintoxicación de otros medicamentos), moxifloxacino (un antibiótico), antipsicóticos (usados para enfermedades mentales graves). Esto es porque Suprefact Depot puede incrementar el riesgo de padecer problemas del ritmo cardiaco cuando se utiliza con estos medicamentos.
Embarazo, lactancia y fertilidad
Suprefact Depot es un medicamento que sólo deben utilizar los hombres. No debe ser utilizado por mujeres.
Conducción y uso de máquinas
Después de tomar este medicamento, usted podría sufrir algún efecto adverso. Alguno de estos efectos adversos (como por ejemplo mareo) puede afectar a la capacidad para concentrarse y a su tiempo de reacción. Si esto sucede, tenga cuidado durante la conducción, utilización de herramientas o máquinas, o durante cualquier trabajo que requiera un nivel alto de atención.
3. Cómo usar Suprefact Depot
El contenido de una jeringa precargada (que contiene 3 implantes cilíndricos con una dosis final de 9,45 mg de buserelina) se inyecta bajo la piel (subcutánea) en el área del estómago, cada 3 meses. Este tiempo se puede aumentar hasta 3 semanas más.
Se debe desinfectar la zona de la inyección. Normalmente, la inyección ha de ser administrada por un médico o enfermera. Antes de su uso, el implante debe de estar a temperatura ambiente. Podría aplicarse un anestésico para aliviar el dolor de la inyección del implante. Siga las indicaciones de su médico acerca de cuándo debe usar Suprefact Depot y el tiempo que tiene que transcurrir entre cada inyección.
Análisis de sangre
- Su médico le realizará análisis de sangre para comprobar si este medicamento está funcionando bien.
Si usa más Suprefact Depot del que debe
Es poco probable que su médico o enfermera le administre más medicamento del que debiera. Si se usa más cantidad de la que se debiera podría sentir debilidad, nerviosismo, mareo o náuseas. También podría sentir dolor de cabeza, sofocos, dolor de estómago, hinchazón (edema) en los tobillos y en las piernas, aumento de las mamas, o reacciones en el lugar donde le han inyectado este medicamento.
Su médico le dará el tratamiento adecuado para estos efectos adversos.
En caso de sobredosis, consulte a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20 indicando el medicamento y la cantidad utilizada.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermera.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si sufre alguno de los efectos adversos, es importante que informe a su médico antes del próximo tratamiento con Suprefact Depot.
Si experimenta alguna reacción alérgica grave tal como falta de aliento o shock, por favor contacte inmediatamente con su médico ya que podría ser necesario extraer el implante.
Efectos adversos que podrían ocurrir al inicio de su tratamiento
Al comienzo de su tratamiento podría aumentar la cantidad de hormonas sexuales que su cuerpo produce y usted podría notar un empeoramiento temporal de los síntomas. Por ejemplo, podría padecer dolor en los huesos, debilidad muscular en las piernas, problemas al orinar, retención de líquidos o problemas de coagulación en los pulmones. Para prevenir esto generalmente se administran otros medicamentos, como por ejemplo acetato de ciproterona. El tratamiento con ciproterona debe continuarse durante 3-4 semanas después de recibir Suprefact Depot. Después de este tiempo, los niveles de testosterona generalmente disminuyen hasta los valores deseados en respuesta a Suprefact Depot.
Informe a su médico o farmacéutico si alguno de los siguientes efectos adversos es grave o dura más de unos días:
Frecuentes (pueden afectar hasta 1 de cada 10 pacientes)
- Pérdida del apetito sexual (libido)
- Dificultad en la erección
- Dolor de cabeza
- Sofocos
- Disminución del tamaño de los testículos (denominado atrofia testicular)
- Dolor u otras reacciones en el lugar de inyección (tales como enrojecimiento o hinchazón)
- Cambios de humor, depresión (tratamiento a largo plazo).
Poco frecuentes (pueden afectar hasta 1 de cada 100 pacientes)
- Reacciones alérgicas como erupciones de la piel que podrían ser de color rojo y picar (incluyendo urticaria)
- Sensación de sueño y cansancio
- Sensación de mareo
- Estreñimiento
- Aumento de las mamas
- Acúmulo de líquido (edema) alrededor de los tobillos y piernas
- Aumento de las enzimas producidas en el hígado que se pone de manifiesto en los resultados de algunos análisis de sangre
- Cambios en el peso
- Cambios de humor, depresión (tratamiento a corto plazo).
Raros (pueden afectar hasta 1 de cada 1000 pacientes)
- Reacciones alérgicas graves como por ejemplo falta de aliento
- Sensación de nerviosismo, estrés, e inestabilidad emocional. Además, dificultad para dormir y problemas de memoria o concentración
- Latidos cardiacos rápidos o irregulares (palpitaciones), aumento de la tensión arterial en personas que la tienen de por sí alta (hipertensión)
- Sensación de mareo (náuseas y vómitos) o diarrea
- Aumento o disminución del cabello y el vello corporal
- Cambios en los lípidos de la sangre y aumento de la bilirrubina que se pone de manifiesto en los resultados de algunos análisis de sangre.
Muy raros (pueden afectar hasta 1 de cada 10.000 pacientes)
- Reacciones alérgicas graves con shock
- Aumento de la sed, cambios en el apetito, bajada de los niveles de tolerancia a la glucosa (en pacientes diabéticos podría producirse una pérdida del control diabético)
- Zumbidos en los oídos (tinnitus) y trastornos auditivos
- Alteraciones de la vista como visión borrosa y sensación de presión en el ojo
- Molestias o dolor en músculos y huesos
- Deterioro del bienestar general
- Disminución del número de células de la sangre que podría provocar anomalías en los resultados de los análisis de sangre y/o hematomas (moratones)
- Aumento del tamaño de tumores “benignos” en la glándula pituitaria o aumento temporal del dolor tumoral.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- Cambios en el electrocardiograma ECG (prolongación del intervalo QT).
Con otras presentaciones que contienen buserelina también se ha observado sensaciones anómalas en piel como por ejemplo hormigueo.
Este grupo de medicamentos (denominados análogos LHRH) pueden provocar una disminución de la densidad del hueso, osteoporosis y un aumento del riesgo de fracturas de hueso. La posibilidad de la fractura de huesos aumenta a lo largo del tratamiento. Los análogos LHRH podrían aumentar el riesgo de enfermedad cardiovascular (como ataque al corazón y accidente cerebrovascular), diabetes o anemia (disminución en el número de glóbulos rojos que provoca que usted se sienta cansado).
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Suprefact Depot
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2ºC y 8ºC). Puede conservarse a una temperatura máxima de 25 °C durante un máximo de 7 días.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Suprefact Depot
El principio activo de Suprefact Depot es buserelina. Cada jeringa está precargada con 1 implante compuesto de 3 cilindros, con una dosis final de 9,9 de acetato de buserelina. Esto equivale a 9,45 mg de buserelina.
El otro componente es Poli (D, L-láctico-co-glicólido) con una proporción de 75:25 de láctico-glicólido.
Aspecto del producto y contenido del envase
Un envase contiene 1 ó 2 jeringas estériles precargadas.
Cada jeringa contiene 3 implantes cilíndricos de color crema.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
CHEPLAPHARM Arzneimittel GmbH
Ziegelhof 24
17489 Greifswald
Alemania
Responsable de la fabricación
Sanofi Aventis Deutschland GmbH
Brüningstraße 50 – Frankfurt am Main
65926 Alemania
Representante Local
Laboratorios Rubió, S.A.
Industria, 29
Pol. Ind. Comte de Sert
08755 Castellbisbal (Barcelona)
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria : Suprefact Depot - Implantat Für 3 Monate
Bélgica: Suprefact Depot 9.45 mg Implant
Dinamarca: Suprefact Depot
Finlandia: Suprefact Depot 9.45 mg implantaatti
Francia: Trigonist 9.45 mg implant pour voie sous-cutanée
Alemania: Profact Depot 9,45 mg 3-Monatsimplantat
Italia: Suprefact depot 3 Mesi
Luxemburgo: Suprefact Depot 9.45 mg Implant
Holanda: Suprefact Depot 3 Maanden, implantatiestift 9.45 mg
Portugal: Suprefact Depot 3 Meses
Suecia: Suprefact Depot 9.45 mg implantat
Reino Unido: Suprefact Depot 9.45 mg implant, for subcutaneous route
Fecha de la última revisión de este prospecto:Junio 2015
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
Esta información está destinada únicamente a profesionales del sector sanitario.
1NOMBRE DEL MEDICAMENTO
Suprefact Depot 9,45 mg implante, por vía subcutánea
2POSOLOGÍA Y FORMA DE ADMINISTRACIÓN
Una jeringa precargada con 1 implante contiene 3 cilindros, que se inyectan bajo la piel del abdomen cada tres meses. Es importante mantener el ritmo de administración cada tres meses de forma regular, sin embargo el intervalo de inyección podría ocasionalmente prolongarse hasta 3 semanas. Antes de la inyección puede administrase un anestésico local.
Antes de su uso, el implante debe de estar a temperatura ambiente.
Advertencia. Para evitar la caída de los 3 cilindros del implante de la aguja de inyección (A), mantener el aplicador en posición vertical hasta inmediatamente antes de la punción, con la aguja apuntando hacia arriba.
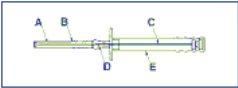
A: Aguja
B: Capuchón protector de la aguja
C: Émbolo
D: Implante
E: Funda protectora del émbolo
- Después de abrir el estuche y sacar el aplicador de su envase, comprobar que los 3 cilindros del implante están situados en la ventanilla del aplicador. En caso necesario, golpear levemente con el dedo el capuchón protector de la jeringa con objeto de recolocarlos en la ventanilla. Una vez abierto el envase, el aplicador debe utilizarse inmediatamente.
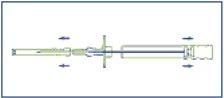
- Desinfectar la zona de la inyección situada en un lado de la pared abdominal. Primero sacar la funda protectora del émbolo (E), y entonces retirar el capuchón protector de la aguja de inyección (B).

- Pellizcar un pliegue de piel e insertar la aguja aproximadamente 3 cm (algo más de una pulgada) en el tejido subcutáneo. Mantener el aplicador en posición horizontal en el momento antes de la punción o con la punta de la aguja ligeramente orientada hacia arriba. Retirar el aplicador 1-2 cm aproximadamente, antes de la inyección de los cilindros.
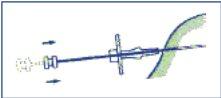
- Inyectar los tres cilindros del implante en el tejido subcutáneo empujando completamente el émbolo. Comprimir el canal de punción mientras se retira la aguja de forma que los 3 cilindros del implante queden retenidos en el tejido.

- Para asegurarse de que los tres cilindros del implante han sido inyectados, comprobar que el extremo del émbolo sea visible en el extremo de la aguja.
Se recomienda la administración de un antiandrógeno 5 días antes del inicio del tratamiento con Suprefact Depot.
3DATOS FARMACÉUTICOS
3.1Lista de excipientes
Poli (D, L-láctido-co-glicólido)
3.2Incompatibilidades
No aplicable dado que el producto se presenta en un aplicador especial.
3.3Período de validez
3 años.
3.4Precauciones especiales de conservación
Conservar en nevera (entre 2ºC y 8ºC). Puede conservarse a una temperatura máxima de 25 °C durante un máximo de 7 días.
3.5Naturaleza y contenido del envase
Jeringa precargada con un implante cilíndrico compuesto por tres cilindros, alojados en un aplicador desechable de propionato de celulosa y acero inoxidable precintado en una bolsa de lámina compuesta de polietileno tereftalato, aluminio y polietileno de baja densidad.
Presentaciones: 1 ó 2 jeringas precargadas por envase.
Puede que solamente estén comercializados algunos tamaños de envases.
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia341.13 EUR
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SUPREFACT DEPOT 9,45 mg IMPLANTEForma farmacéutica: INYECTABLE, 1 mg/mlPrincipio activo: buserelinFabricante: Cheplapharm Arzneimittel GmbhRequiere recetaForma farmacéutica: INYECTABLE, 42 mgPrincipio activo: leuprorelinFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE, 0,1 mgPrincipio activo: triptorelinaFabricante: Ipsen Pharma S.A.Requiere receta
Médicos online para SUPREFACT DEPOT 9,45 mg IMPLANTE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SUPREFACT DEPOT 9,45 mg IMPLANTE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes












