
SIRTURO 100 MG COMPRIMIDOS

Cómo usar SIRTURO 100 MG COMPRIMIDOS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
SIRTURO 100 mg comprimidos
Bedaquilina
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque
contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es SIRTURO y para qué se utiliza
- Qué necesita saber antes de empezar a tomar SIRTURO
- Cómo tomar SIRTURO
- Posibles efectos adversos
- Conservación de SIRTURO
- Contenido del envase e información adicional
1. Qué es SIRTURO y para qué se utiliza
SIRTURO contiene el principio activo bedaquilina.
SIRTURO es un tipo de antibiótico. Los antibióticos son medicamentos que destruyen bacterias
causantes de enfermedades.
SIRTURO se utiliza para tratar la tuberculosis que afecta a los pulmones cuando la enfermedad se ha hecho resistente a otros antibióticos. Es lo que se denomina tuberculosis pulmonar multirresistente.
SIRTURO se debe utilizar siempre conjuntamente con otros medicamentos para el tratamiento de la tuberculosis.
Se utiliza en adultos de 18 o más años de edad.
2. Qué necesita saber antes de empezar a tomar SIRTURO
No tome SIRTURO:
- si es alérgico a bedaquilina o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6). No tome SIRTURO si lo anterior se aplica en su caso. Si no está seguro, consulte con su médico o farmacéutico antes de tomar SIRTURO.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a tomar SIRTURO, si:
- presenta alguna anomalía en el electrocardiograma (ECG) o insuficiencia cardiaca;
- tiene antecedentes personales o familiares de un problema cardiaco llamado “síndrome del QT prolongado congénito”;
- tiene una disminución de la función de la glándula tiroidea. Esto se puede observar en un análisis de sangre;
- tiene una enfermedad hepática o si bebe alcohol habitualmente;
- tiene una infección por el virus de la inmunodeficiencia humana (VIH).
Si alguna de las situaciones anteriores se aplica en su caso (o si tiene dudas), hable con su médico, farmacéutico o enfermero antes de tomar SIRTURO.
Niños y adolescentes
No administre este medicamento a niños y adolescentes (menores de 18 años), puesto que no se ha estudiado en este grupo de edad.
Toma de SIRTURO con otros medicamentos
Otros medicamentos pueden afectar a SIRTURO. Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
A continuación se indican ejemplos de medicamentos que los pacientes con tuberculosis multirresistente pueden tomar y que pueden interaccionar potencialmente con SIRTURO:
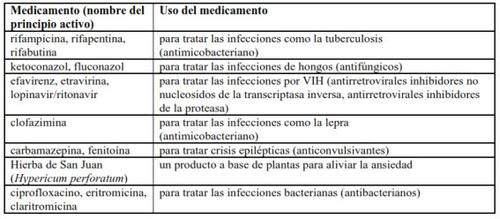
Toma de SIRTURO con alcohol
No debe ingerir alcohol mientras esté tomando SIRTURO.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Es posible que se sienta mareado después de tomar SIRTURO. Si esto sucede, no conduzca ni utilice maquinaria.
SIRTURO contiene lactosa monohidrato
SIRTURO contiene “lactosa” (un tipo de azúcar). Si tiene intolerancia a algunos azúcares o no puede digerirlos, hable con su médico antes de tomar este medicamento.
3. Cómo tomar SIRTURO
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
SIRTURO debe utilizarse siempre conjuntamente con otros medicamentos para el tratamiento de la tuberculosis. Su médico decidirá qué otros medicamentos debe tomar con SIRTURO.
Qué cantidad debe tomar
Tome SIRTURO durante un periodo de 24 semanas.
Primeras 2 semanas:
- Tome 400 mg (4 comprimidos de 100 mg) una vez al día.
De la semana 3 a la semana 24:
- Tome 200 mg (2 comprimidos de 100 mg) una vez al día durante 3 días a la semana únicamente.
- Debe transcurrir un intervalo mínimo de 48 horas entre dosis siempre que tome SIRTURO. Por ejemplo, puede tomar SIRTURO los Lunes, Miércoles y Viernes todas las semanas a partir de la semana 3.
Es posible que tenga que seguir tomando sus otros medicamentos para la tuberculosis durante un período superior a 6 meses. Consulte a su médico o farmacéutico.
Cómo tomar este medicamento
- Tome SIRTURO con alimentos. Los alimentos son importantes para conseguir los niveles
- adecuados del medicamento en su cuerpo.
- Trague los comprimidos enteros con agua.
Si toma más SIRTURO del que debe
Si toma más SIRTURO de lo que debe, informe a su médico inmediatamente. Lleve consigo el envase del medicamento.
Si olvidó tomar SIRTURO
Durante la primeras 2 semanas
- Omita la dosis olvidada y tome la dosis siguiente a su hora habitual.
- No tome una dosis doble para compensar las dosis olvidadas.
A partir de la semana 3
- Tome la dosis olvidada de 200 mg lo antes posible.
- Reanude la pauta de tres veces a la semana
Si se olvida de tomar una dosis y no está seguro de lo que debe hacer, hable con su médico o
farmacéutico.
Si interrumpe el tratamiento con SIRTURO
No deje de tomar SIRTURO sin hablar antes con su médico.
La omisión de dosis o la no finalización del ciclo completo de tratamiento puede:
- hacer que el tratamiento sea ineficaz y que su tuberculosis empeore y;
- aumentar la probabilidad de que la bacteria se haga resistente al medicamento. Esto puede hacer que su enfermedad no responda al tratamiento con SIRTURO o a otros medicamentos en el futuro.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- dolor de cabeza
- dolor de las articulaciones
- sensación de mareo
- sentirse o estar enfermo (náuseas o vómitos)
Frecuentes(pueden afectar hasta a 1 de cada 10 personas):
- diarrea
- elevación de las enzimas hepáticas (aparece en los análisis de sangre)
- dolor o hipersensibilidad muscular, no causada por ejercicio
- anomalía detectada en el electrocardiograma llamada “prolongación del intervalo QT”. Informe en seguida a su médico si sufre un desmayo.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V . Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de SIRTURO
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja después de CAD.
La fecha de caducidad es el último día del mes que se indica.
Conservar SIRTURO en el embalaje o envase original para protegerlo de la luz.
Este medicamento puede tener riesgo para el medioambiente. Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de SIRTURO
- El principio activo es bedaquilina. Cada comprimido contiene fumarato de bedaquilina equivalente a 100 mg de bedaquilina.
- Los demás componentes son: sílice coloidal anhidra, croscarmelosa sódica, hipromelosa, lactosa monohidrato, estearato de magnesio, almidón de maíz, celulosa microcristalina, polisorbato 20.
Aspecto del producto y contenido del envase
Comprimido no recubierto, de color blanco a blanquecino, redondo, biconvexo, de 11 mm de diámetro, con la inscripción “T” sobre “207” grabada en una cara y “100” en la otra.
Frasco de plástico con 188 comprimidos.
Envase que contiene 4 tiras de blíster (que contienen 6 comprimidos por tira).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Bélgica
Responsable de la fabricación
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
36
Belgium
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Janssen-Cilag NV Antwerpseweg 15-17 B-2340 Beerse Tel/Tél: +32 14 64 94 11 | Lietuva UAB "JOHNSON & JOHNSON" Konstitucijos pr. 21C LT-08130 Vilnius Tel: +370 5 278 68 88 |
???????? „??????? & ??????? ????????” ???? ?.?. ??????? 4 ?????? ???? ?????, ?????? 4 ????? 1766 ???.: +359 2 489 94 00 | Luxembourg/Luxemburg Janssen-Cilag NV Antwerpseweg 15-17 B-2340 Beerse Belgique/Belgien Tél/Tel: +32 14 64 94 11 |
Ceská republika Janssen-Cilag s.r.o. Walterovo námestí 329/1 CZ-158 00 Praha 5 – Jinonice Tel: +420 227 012 227 | Magyarország Janssen-Cilag Kft. Nagyenyed u. 8-14 H-Budapest, 1123 Tel.: +36 1 884 2858 |
Danmark Janssen-Cilag A/S Bregnerødvej 133 DK-3460 Birkerød Tlf: +45 45 94 82 82 | Malta AM MANGION LTD. Mangion Building, Triq Gdida fi Triq Valletta MT-Hal-Luqa LQA 6000 Tel: +356 2397 6000 |
Deutschland Janssen-Cilag GmbH Johnson & Johnson Platz 1 D-41470 Neuss Tel: +49 2137 955-955 | Nederland Janssen-Cilag B.V. Graaf Engelbertlaan 75 NL-4837 DS Breda Tel: +31 76 711 1111 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Lõõtsa 2 EE-11415 Tallinn Tel: +372 617 7410 | Norge Janssen-Cilag AS Postboks 144 NO-1325-Lysaker Tlf: +47 24 12 65 00 |
Ελλ?da Janssen-Cilag Faρµaκeυtικ? Α.Ε.Β.Ε. Λeωf?ρος Ειρ?νης 56 GR-151 21 Πe?κη, Αθ?νa Tηλ: +30 210 80 90 000 | Österreich Janssen-Cilag Pharma GmbH Vorgartenstraße 206B A-1020 Wien Tel: +43 1 610 300 |
España Janssen-Cilag, S.A. Paseo de las Doce Estrellas, 5-7 E-28042 Madrid Tel: +34 91 722 81 00 | Polska Janssen-Cilag Polska Sp. z o.o. ul. Ilzecka 24 PL-02-135 Warszawa Tel.: +48 22 237 60 00 |
France Janssen-Cilag 1, rue Camille Desmoulins, TSA 91003 F-92787 Issy Les Moulineaux, Cedex 9 Tél: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Lagoas Park, Edifício 9 2740-262 PORTO SALVO PORTUGAL Tel: +351 214 368 600 |
Hrvatska Johnson & Johnson S.E. d.o.o. Oreškoviceva 6h 10010 Zagreb Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Str. Tipografilor nr. 11-15 Cladirea S-Park, Corp B3-B4, Etaj 3 013714 Bucuresti, ROMÂNIA Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Barnahely Ringaskiddy IRL – Co. Cork P43 FA46 Tel: +353 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Šmartinska cesta 53 SI-1000 Ljubljana Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB c/o Vistor hf. Hörgatúni 2 IS-210 Garðabær Sími: +354 535 7000 | Slovenská republika Johnson & Johnson s.r.o. CBC III, Karadžicova 12 SK-821 08 Bratislava Tel: +421 232 408 400 |
Italia Janssen-Cilag SpA Via M.Buonarroti, 23 I-20093 Cologno Monzese MI Tel: +39 02 2510 1 | Suomi/Finland Janssen-Cilag Oy Vaisalantie/Vaisalavägen 2 FI-02130 Espoo/Esbo Puh/Tel: +358 207 531 300 |
Κ?pρος Βaρν?ßaς Χatζηpaνaγ?ς Λtd, Λeωf?ρος Gι?ννου Κρaνιdι?tη 226 Λatsι? CY-2234 Λeυκωs?a Τηλ: +357 22 207 700 | Sverige Janssen-Cilag AB Box 4042 SE-16904 Solna Tel: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Mukusalas iela 101 Riga, LV-1004 Tel: +371 678 93561 | United Kingdom Janssen-Cilag Ltd. 50-100 Holmers Farm Way High Wycombe Buckinghamshire HP12 4EG - UK Tel: +44 1 494 567 444 |
Fecha de la última revisión de este prospecto
Este medicamento se ha autorizado con una «aprobación condicional».
Esta modalidad de aprobación significa que se espera obtener más información de este medicamento. La Agencia Europea de Medicamentos revisará la información nueva de este medicamento al menos una vez al año y este prospecto se actualizará cuando sea necesario.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SIRTURO 100 MG COMPRIMIDOSForma farmacéutica: COMPRIMIDO, 100 mgPrincipio activo: bedaquilineFabricante: Janssen-Cilag International N.VRequiere recetaForma farmacéutica: COMPRIMIDO, 50 mgPrincipio activo: DelamanidFabricante: Otsuka Novel Products GmbhRequiere recetaForma farmacéutica: COMPRIMIDO, 400 mgPrincipio activo: ethambutolFabricante: Teofarma S.R.L.Requiere receta
Médicos online para SIRTURO 100 MG COMPRIMIDOS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SIRTURO 100 MG COMPRIMIDOS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















