
SERETIDE ACCUHALER 50 microgramos/250 microgramos/INHALACIÓN, POLVO PARA INHALACIÓN.


Cómo usar SERETIDE ACCUHALER 50 microgramos/250 microgramos/INHALACIÓN, POLVO PARA INHALACIÓN.
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Seretide Accuhaler 50 microgramos/250 microgramos/inhalación, polvo para inhalación
salmeterol/propionato de fluticasona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Seretide y para qué se utiliza
- Qué necesita saber antes de empezar a usar Seretide
- Cómo usar Seretide
- Posibles efectos adversos
- Conservación de Seretide
- Contenido del envase e información adicional
1. Qué es Seretide y para qué se utiliza
Seretide contiene dos principios activos, salmeterol y propionato de fluticasona:
- Salmeterol es un broncodilatador de larga duración. Los broncodilatadores ayudan a mantener abiertas las vías respiratorias en el pulmón, haciendo más fácil la entrada y salida de aire. Los efectos duran al menos 12 horas.
- Propionato de fluticasona es un corticosteroide que disminuye la inflamación e irritación de los pulmones.
El médico le ha prescrito este medicamento para ayudar a prevenir los problemas respiratorios tales como:
- Asma.
- Enfermedad Pulmonar Obstructiva Crónica (EPOC). Seretide Accuhaler, en una dosis de 50/500 microgramos, reduce el número de reagudizaciones de los síntomas de EPOC.
Usted debe utilizar Seretide cada día como le ha recomendado su médico. Esto asegurará que la medicación actúe correctamente en el control de su asma o EPOC.
Seretide ayuda a impedir la falta de aliento y sibilancias. Sin embargo, Seretide no se debe utilizar para aliviar un ataque repentino de ahogo o sibilancias. En tal caso, usted tiene que utilizar su medicación de “rescate” de acción rápida, como salbutamol. Debe llevar consigo en todo momento su medicación de rescate de acción rápida.
2. Qué necesita saber antes de empezar a usar Seretide
No use Seretide
- si es alérgico al salmeterol, propionato de fluticasona o al otro componente, lactosa monohidrato.
Advertencias y precauciones
Consulte a su médico antes de comenzar el tratamiento si tiene:
- Alteraciones cardiacas incluyendo latido cardiaco rápido o irregular.
- Hiperactividad tiroidea.
- Tensión arterial elevada.
- Diabetes mellitus (Seretide puede aumentar los niveles de azúcar en sangre).
- Niveles bajos de potasio en sangre.
- Tuberculosis (TB) a día de hoy o en el pasado u otras infecciones del pulmón.
Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Otros medicamentos y Seretide
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, incluyendo aquellos medicamentos para el asma o los adquiridos sin receta. La razón es que, en algunos casos, Seretide no debe ser administrado junto con otros medicamentos.
Informe a su médico si usted toma alguno de los siguientes medicamentos, antes de empezar a utilizar Seretide:
- β bloqueantes (tales como atenolol, propranolol y sotalol). Los β bloqueantes son utilizados en su mayor parte para tratar la hipertensión u otras afecciones cardiacas.
- Medicamentos para tratar infecciones (como ketoconazol, itraconazol y eritromicina) incluyendo algunos medicamentos para el VIH (como ritonavir, cobicistat). Algunos de estos medicamentos pueden aumentar la cantidad de propionato de fluticasona o salmeterol en su organismo. Esto puede aumentar su riesgo de padecer efectos adversos con Seretide, incluyendo latidos del corazón irregulares, o pueden empeorar los efectos adversos, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos.
- Corticosteroides (orales o inyectables). Si usted ha tomado estos medicamentos recientemente, puede aumentar el riesgo de que este medicamento afecte a su glándula suprarrenal.
- Diuréticos, también conocidos como medicamentos para orinar, usados para tratar la tensión arterial alta.
- Otros broncodilatadores (como salbutamol).
- Medicamentos que contienen xantina. Se usan a menudo para tratar el asma.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
No es probable que Seretide afecte a la capacidad de conducir o usar máquinas.
Seretide Accuhaler contiene lactosa
Seretide Accuhaler contiene hasta 12,5 miligramos de lactosa monohidrato en cada dosis. La cantidad de lactosa en este medicamento normalmente no causa problemas en gente intolerante a la lactosa. Puede provocar reacciones alérgicas en pacientes con alergia a la proteína de la leche de vaca. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de usar este medicamento.
3. Cómo usar Seretide
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
- Utilice Seretide todos los días, hasta que su médico le indique que deje de hacerlo. No tome más de la dosis recomendada. En caso de duda, consulte a su médico o farmacéutico.
- No deje de tomar Seretide ni reduzca su dosis sin hablar antes con su médico.
- Seretide debe inhalarse a través de la boca hasta los pulmones.
- Es posible que no pueda saborear o sentir el polvo en su lengua, incluso si ha utilizado el Accuhaler correctamente.
Para asma
Adultos y adolescentes de 12 años en adelante
- Seretide 50/100 Accuhaler - Una inhalación dos veces al día
- Seretide 50/250 Accuhaler - Una inhalación dos veces al día
- Seretide 50/500 Accuhaler - Una inhalación dos veces al día
Niños de 4 a 12 años de edad
- Seretide 50/100 Accuhaler - Una inhalación dos veces al día
Seretide no está recomendado para uso en niños menores de 4 años de edad.
Para adultos con Enfermedad Pulmonar Obstructiva Crónica (EPOC)
- Seretide 50/500 Accuhaler - Una inhalación dos veces al día
Sus síntomas pueden llegar a estar bien controlados utilizando Seretide dos veces al día. Si es así, su médico podrá decidir disminuir su dosis a una vez al día. La dosis puede cambiar a:
- una vez por la noche si tiene síntomas nocturnos,
- una vez por la mañana si tiene síntomas diurnos.
Es muy importante que siga las instrucciones de su médico sobre cuántas aplicaciones y con qué frecuencia debe tomar su medicación.
Si está utilizando Seretide para tratar el asma, su médico querrá vigilar regularmente sus síntomas.
Si su asma empeora o tiene mayor dificultad para respirar, acuda a su médico enseguida. Puede notar más pitos o sensación de ahogo más a menudo o que tenga que utilizar su medicación de rescate de acción rápida con más frecuencia. Si le ocurre cualquiera de estas cosas, debe continuar utilizando Seretide, pero no aumente el número de aplicaciones. Su enfermedad respiratoria puede empeorar y enfermar gravemente. Acuda a su médico, puesto que puede que necesite un tratamiento adicional.
Instrucciones de uso
- Su médico, enfermero o farmacéutico deberán enseñarle cómo utilizar su inhalador. Periódicamente deberían verificar cómo lo utiliza. El no utilizar Seretide Accuhaler apropiadamente ni como se le ha prescrito, puede tener como resultado que su asma o EPOC no mejore como debiera.
- Este Accuhaler contiene alvéolos que contienen Seretide en polvo.
- Hay un contador de dosis en la parte superior del Accuhaler que señala cuántas dosis quedan. Cuenta hacia atrás, hasta 0. Los números de 5 a 0 aparecerán en rojo para advertirle que quedan pocas dosis. Una vez que el contador marca 0, su inhalador está vacío.
Utilización de su inhalador
- Para abrir el Accuhaler, coger con una mano la carcasa externa y colocar el dedo pulgar de la otra mano en el hueco reservado para ello. Empujar con el dedo, alejándolo de usted, hasta donde llegue. Oirá un “clic”. Esto abrirá un pequeño orificio en la boquilla.
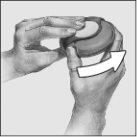
- Mantener el dispositivo con la boquilla hacia usted. Puede cogerlo con su mano derecha o izquierda. Deslizar la palanca, alejándola. Oirá un “clic”. Esto colocará la dosis de medicamento en la boquilla.
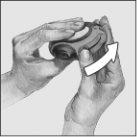
Cada vez que la palanca se echa hacia atrás, se abre un alvéolo y el polvo queda preparado para ser inhalado. No jugar con la palanca pues se abren los alvéolos y se desperdicia medicamento.
- Mantener el Accuhaler alejado de la boca. Expulsar el aire lo que razonablemente se pueda. No respirar dentro del Accuhaler.
- Colocar la boquilla en los labios; tomar aire progresiva e intensamente a través del Accuhaler, no por la nariz.
Sacar el Accuhaler de la boca.
Mantener la respiración unos 10 segundos o tanto tiempo como sea posible.
Expulsar el aire lentamente.

- Después enjuague su boca con agua y escúpala y/o cepíllese los dientes. Esto puede ayudarle a prevenir ulceraciones en la boca y a tener ronquera.
- Para cerrar el Accuhaler, deslizar con el dedo pulgar la palanca hacia usted todo lo que pueda. Oirá un “clic”.
La palanca automáticamente volverá a su posición original.
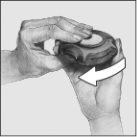
El Accuhaler está ahora preparado para volverlo a utilizar.
Al igual que en todos los inhaladores, los cuidadores se deben asegurar de que los niños que estén usando Seretide Accuhaler, usen correctamente la técnica de inhalación descrita anteriormente.
Limpieza de su inhalador
Para limpiarlo, pasar un pañuelo seco por la pieza bucal del Accuhaler.
Si usa más Seretide del que debe
Es muy importante usar el inhalador tal y como le han indicado. Si accidentalmente usted ha tomado una dosis mayor de la recomendada, consulte a su médico o farmacéutico. Puede notar que su corazón late más rápido de lo normal y sentir temblores. También puede tener mareo, dolor de cabeza, debilidad muscular y dolor en las articulaciones.
Si usted ha utilizado grandes dosis durante largos periodos de tiempo, usted debe pedir consejo a su médico o farmacéutico. Esto es porque altas concentraciones de Seretide pueden reducir la cantidad de hormonas esteroideas producidas por la glándula suprarrenal.
En caso de sobredosis consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad administrada.
Si olvidó usar Seretide
No tome una dosis doble para compensar las dosis olvidadas. Tome la siguiente dosis a la hora habitual.
Si interrumpe el tratamiento con Seretide
Es muy importante que utilice Seretide todos los días tal como se le ha indicado. Siga tomándolo hasta que su médico le indique que finalice el tratamiento. No interrumpa bruscamente su tratamiento con Seretide.Esto podría hacer que su respiración empeore.
Además, si deja de tomar Seretide de forma repentina o reduce su dosis, podría (muy raramente) causarle problemas en la glándula suprarrenal (insuficiencia suprarrenal), que algunas veces causa efectos adversos.
Estos efectos adversos pueden incluir cualquiera de los siguientes:
- Dolor de estómago.
- Cansancio y pérdida del apetito, sensación de malestar.
- Malestar y diarrea.
- Pérdida de peso.
- Dolor de cabeza o somnolencia.
- Bajos niveles de azúcar en su sangre.
- Hipotensión y convulsiones (ataques).
Cuando su cuerpo se encuentra bajo situaciones de estrés tales como fiebre, traumatismo (p.ej., accidente de tráfico), infección o cirugía, la insuficiencia suprarrenal puede empeorar y podría tener cualquiera de los efectos adversos listados anteriormente.
Si tiene cualquier efecto adverso consulte a su médico o farmacéutico. Para prevenir estos síntomas, su médico puede prescribirle una dosis adicional de corticosteroides en comprimidos durante ese tiempo (como prednisolona).
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, enfermero o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Para reducir la aparición de efectos adversos, su médico le prescribirá la menor dosis de Seretide que controle su asma o Enfermedad Pulmonar Obstructiva Crónica (EPOC).
Reacciones alérgicas: puede notar que su respiración, de repente, empeora inmediatamentedespués de utilizar Seretide. Puede sufrir pitos y tos o falta de aliento. También puede notar picor, erupción (urticaria) e hinchazón (generalmente de la cara, labios, lengua o garganta). También puede sentir, de repente, que su corazón late muy rápido, sentir que pierde el conocimiento y mareo (que puede llevar al colapso o pérdida de la conciencia). Si sufre cualquiera de estos efectos o siaparecen de repente después de utilizar Seretide, deje de tomar Seretide y avise a su médico enseguida. Las reacciones alérgicas a Seretide son poco frecuentes (pueden afectar hasta 1 de cada 100 personas).
Neumonía (infección de los pulmones) en pacientes con EPOC (efecto adverso frecuente)
Informe a su médicosi usted tiene cualquiera de los siguientes síntomas mientras inhala Seretide, podrían ser síntomas de una infección pulmonar:
- Fiebre o escalofríos.
- Aumento de la producción de moco, cambio en el color del moco.
- Aumento de la tos o aumento de dificultades para respirar.
A continuación se enumeran otros efectos adversos:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Dolor de cabeza, que normalmente mejora al continuar con el tratamiento.
- Se ha notificado un aumento del número de resfriados en pacientes con Enfermedad Pulmonar Obstructiva Crónica (EPOC).
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Candidiasis (picor, aparición de úlceras de color amarillo crema) en la boca y la garganta. También dolor en la lengua, voz ronca e irritación de garganta. Enjuagar la boca con agua y escupirla y/o cepillarse los dientes inmediatamente después de cada dosis de medicamento puede ayudarle. Para el tratamiento de la candidiasis, su médico puede prescribirle medicación antifúngica (para el tratamiento de infecciones por hongos).
- Dolor, inflamación en las articulaciones y dolor muscular.
- Calambres musculares.
Los siguientes efectos adversos se han notificado en pacientes con Enfermedad Pulmonar Obstructiva Crónica (EPOC):
- Cardenales y fracturas.
- Inflamación de los senos (sensación de tensión o congestión en la nariz, mejillas y detrás de los ojos, a veces con un dolor pulsátil).
- Reducción de los niveles de potasio en sangre (puede sentir latidos del corazón irregulares, debilidad muscular, calambres).
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- Aumento de los niveles de azúcar (glucosa) en sangre (hiperglucemia). Si padece diabetes, será necesario controlar sus niveles de azúcar en sangre con mayor frecuencia y ajustar su tratamiento diabético habitual en caso de necesidad.
- Cataratas (opacidad del cristalino del ojo).
- Ritmo cardiaco muy rápido (taquicardia).
- Sentir temblores y un ritmo cardiaco rápido o irregular (palpitaciones). Estos efectos adversos son habitualmente inofensivos y disminuyen cuando se continúa con el tratamiento.
- Dolor en el pecho.
- Sensación de preocupación (ocurre principalmente en niños).
- Trastornos del sueño.
- Erupción cutánea.
- Erupción alérgica en la piel.
Raros (pueden afectar hasta 1 de cada 1000 personas)
- Dificultad para respirar (o sibilancias) que empeora justo después de utilizar Seretide.Si esto sucede, deje de utilizar Seretide.Utilice su inhalador de "rescate" de acción rápida para mejorar su respiración y avise a su médico enseguida.
- Seretide puede aumentar la producción normal de hormonas esteroideas, particularmente si ha estado tomando altas dosis durante largos periodos de tiempo. Los efectos incluyen:
- Retraso en el crecimiento en niños y adolescentes.
- Disminución de la densidad mineral ósea.
- Glaucoma.
- Aumento de peso.
- Cara redondeada (en forma de luna llena) (Síndrome de Cushing).
Su médico vigilará regularmente cualquiera de estos efectos adversos y se cerciorará de que está tomando la dosis más baja de Seretide para controlar su asma.
- Cambios en el comportamiento, tales como hiperactividad e irritabilidad (estos efectos ocurren fundamentalmente en niños).
- Latidos del corazón irregulares o que el corazón tenga latidos extra (arritmias). Consulte a su médico, pero no deje de tomar Seretide a menos que su médico le diga que lo haga.
- Infección causada por hongos en el esófago (garganta), que puede causar dificultad para tragar.
No conocida: la frecuencia no puede estimarse a partir de los datos disponibles
- Depresión o agresividad. Es más probable que estos efectos aparezcan en niños.
- Visión borrosa.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Seretide
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice Seretide después de la fecha de caducidad que aparece en la etiqueta y en el estuche después de CAD. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 30 ºC.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Seretide
- Cada dosis predispensada contiene 50 microgramos de salmeterol (como xinafoato de salmeterol) y 250 microgramos de propionato de fluticasona.
- El otro componente es lactosa monohidrato (contiene proteínas de leche).
Aspecto del producto y contenido del envase
Seretide Accuhaler contiene una tira de alvéolos. Los alvéolos protegen el polvo para inhalación de los efectos atmosféricos.
Cada dosis es pre-dispensada.
Los dispositivos se encuentran en envases de:
- 1 Accuhaler x 28 inhalaciones o
- 1, 2, 3 o 10 Accuhaler x 60 inhalaciones cada uno.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel: +34 900 202 700
Responsable de la fabricación:
Glaxo Wellcome Production
Zone Industrielle nº 2, 23 Rue Lavoisier, La Madeleine, 27000 Evreux, Francia
Tel: + 33 2 3223 5500
Fax: +33 2 3223 5558
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria Seretide Diskus
Bélgica Seretide Diskus
Croacia Seretide Diskus
Chipre Seretide Diskus
República Checa Seretide Diskus
Dinamarca Seretide
Estonia Seretide Diskus
Finlandia Seretide Diskus
Francia Seretide Diskus
Alemania atmadisc Diskus
Grecia Seretide Diskus
Hungría Seretide Diskus
Islandia Seretide
Irlanda Seretide Diskus
Italia Seretide Diskus
Luxemburgo Seretide Diskus
Malta Seretide Diskus
Países Bajos Seretide Diskus
Portugal Seretaide Diskus
Rumanía Seretide Diskus
Eslovaquia Seretide Diskus
España Seretide Accuhaler
Suecia Seretide Diskus
Fecha de la última revisión de este prospecto:julio 2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia41.28 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SERETIDE ACCUHALER 50 microgramos/250 microgramos/INHALACIÓN, POLVO PARA INHALACIÓN.Forma farmacéutica: INHALACIÓN PULMONAR, 50 microgramos/250 microgramosPrincipio activo: salmeterol and fluticasoneFabricante: Sandoz Farmaceutica S.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 50 microgramos/500 microgramosPrincipio activo: salmeterol and fluticasoneFabricante: Sandoz Farmaceutica S.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 50 microgramos /100 microgramosPrincipio activo: salmeterol and fluticasoneFabricante: Zentiva K.S.Requiere receta
Médicos online para SERETIDE ACCUHALER 50 microgramos/250 microgramos/INHALACIÓN, POLVO PARA INHALACIÓN.
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SERETIDE ACCUHALER 50 microgramos/250 microgramos/INHALACIÓN, POLVO PARA INHALACIÓN., sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















