
ROPSINE 7.5 MG/ML SOLUCION INYECTABLE EFG

Cómo usar ROPSINE 7.5 MG/ML SOLUCION INYECTABLE EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Ropsine7,5 mg/ml solución inyectable EFG
Ropivacaína, hidrocloruro
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Ropsine y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ropsine
- Cómo usar Ropsine
- Posibles efectos adversos
- Conservación de Ropsine
- Contenido del envase e información adicional
1. Qué es Ropsine y para qué se utiliza
Ropsine contiene el principio activo hidrocloruro de ropivacaína el cual pertenece a una clase de medicamentos denominados anestésicos locales.
Ropsine 7,5 mg/ml solución inyectable EFG se utiliza en adultos y adolescentes mayores de 12 años para insensibilizar (anestesiar) partes del cuerpo. Se utiliza para detener o aliviar el dolor. Se puede utilizar para:
- Insensibilizar una parte del cuerpo en cirugía, incluyendo el tener un bebé por cesárea.
- Aliviar el dolor durante el parto, después de la cirugía o después de un accidente.
2. Qué necesita saber antes de empezar a usar Ropsine
No useRopsine
- si es alérgico(hipersensible) al hidrocloruro de ropivacaína, a cualquier otro anestésico de tipo amida o a cualquiera de los demás componentes de Ropsine (incluidos en la sección 6),
- si presenta un volumen de sangre disminuido(hipovolemia). Esto le será mesurado por personal sanitario,
- para inyectarlo en un vaso sanguíneopara insensibilizarle una zona específica de su cuerpo,
- para inyectarlo en el cuello del úteropara aliviar el dolor durante el parto.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Ropsine
En niños de hasta 12 años inclusive, pueden ser más apropiadas otras concentraciones (2 mg/ml, 5 mg/ml).
Se debe tener un cuidado especial para evitar cualquier inyecciónde Ropsine directamente en un vaso sanguíneopara prevenir cualquier efecto tóxico inmediato. La inyección no debe realizarse en un área inflamada.
Informe a su médico:
- si tiene una mala condición generaldebido a la edad o a otros factores,
- si tiene problemas de corazón(bloqueo de la conducción cardiaca parcial o completa),
- si tiene un problemade hígadoavanzado,
- si tiene problemasde riñónseveros.
Informe a su médico si tiene cualquiera de estos problemas ya que su médico deberá ajustarle la dosis de Ropsine.
Informe a su médico:
- si padece porfiria aguda(problemas con la generación de los pigmentos rojos de la sangre, a veces resulta en síntomas neurológicos).
Informe a su médico si usted o alguien de su familia padece porfirina ya que su médico puede necesitar utilizar otro anestésico.
Uso deRopsinecon otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Se debe tener precaución si está recibiendo:
- Otros anestésicos locales(por ejemplo, lidocaína) o agentes estructuralmente relacionados con los anestésicos locales de tipo amida, por ejemplo, ciertos medicamentos utilizados para tratar latidos del corazón irregulares (arritmia), tales como la mexiletina o la amiodarona,
- Anestésicos generalesu opiodes, tales como la morfina o la codeína,
- Medicamentos utilizados para tratar la depresión(por ejemplo, fluvoxamina),
- Ciertos antibióticos(por ejemplo, enoxacina).
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento. Se desconoce si el hidrocloruro de ropivacaína afecta al embarazo o si pasa a la leche materna.
Conducción y uso de máquinas
Ropsine puede producir somnolencia y afectar la velocidad de sus reacciones. No conduzca ni utilice herramientas o máquinas después de tomar Ropsine, hasta el día siguiente.
Consulte a su médico o farmacéutico si tiene dudas.
Información importante sobre algunos de los componentes deRopsine
Este medicamento contiene 3 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada ml. Esto equivale al 0,15% de la ingesta diaria máxima de sodio recomendada para un adulto.
3. Cómo usar Ropsine
Método de administración
Ropsine le será administrado por su médico. Se le administrará mediante una inyección.
Dosis
La dosis recomendada dependerá de para qué se está utilizando y también de su salud, edad y peso.
Se debe utilizar la dosis más pequeña que pueda producir un efecto insensibilizador (anestesia) de la zona requerida.
La dosis usual
- para adultosy adolescentes mayores de 12 añosestá entre 2 mg y 300 mgde hidrocloruro de ropivacaína.
- en lactantes y niños (de 0 hasta los 12 años, ambos incluidos)está entre 1-3 mg por cada kilogramode peso corporal.
Duración del tratamiento
La administración de hidrocloruro de ropivacaína por lo general dura entre2 y 10 horasen caso de anestesiaantes de ciertas cirugías y puede tardar hasta 72 horasen caso de alivio del dolor durante o después de la cirugía.
Si se le administra másRopsinedel que debe
Los primeros síntomas de que se le ha administrado más hidrocloruro de ropivacaína del que debiera, normalmente son problemas relacionados con:
- oído y vista,
- adormecimiento alrededor de la boca,
- mareos o desvanecimientos,
- hormigueo,
- trastorno del habla caracterizado por una pobre articulación (disartria),
- rigidez muscular, espasmos musculares, ataques (convulsiones),
- presión arterial baja,
- ritmo cardíaco lento o irregular
Estos síntomas pueden preceder a un paro cardíaco, paro respiratorio o convulsiones graves.
Si usted experimenta alguno de estos síntomas o cree que puede haber recibido demasiadaRopsine, informe a su médico o personal sanitario inmediatamente.
En caso de toxicidad aguda, inmediatamente se tomarán las medidas correctivas apropiadas por el personal sanitario.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Ropsine puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos importantes que se deben tener en cuenta:
Las reacciones alérgicas repentinas y potencialmente mortales (p. ej., anafilaxia, incluido el shock anafiláctico) son raras y afectan de 1 a 10 personas de cada 10 000. Los posibles síntomas incluyen:
- inicio repentino de la erupción, picor o habón (urticaria);
- hinchazón de la cara, los labios, la lengua u otras partes del cuerpo;
- falta de aliento, sibilancias o dificultad para respirar;
- y una sensación de pérdida de la consciencia.
Si cree queRopsinele está provocando una reacción alérgica, informe a su médico o personal sanitario inmediatamente.
Otros efectos adversos posibles:
Muy frecuentes(puede afectar a más de 1 de cada 10 personas)
- Tensión arterial baja (hipotensión). Esto podría hacerle sentir mareado o aturdido.
- Sensación de malestar (náuseas).
Frecuentes(puede afectar hasta 1 de cada 10 personas)
- Dolor de cabeza, hormigueo (parestesias), sensación de mareo.
- Latido del corazón lento o rápido (bradicardia, taquicardia).
- Tensión arterial alta (hipertensión).
- Sensación de malestar (vómitos).
- Dificultades para orinar (retención urinaria).
- Dolor de espalda, temperatura alta, rigidez muscular.
Poco frecuentes(puede afectar hasta 1 de cada 100 personas)
- Ansiedad.
- Algunos síntomas pueden aparecer si la inyección se realizó por error en un vaso sanguíneo, o si se le ha administrado más Ropsine del que debiera (ver también sección 3 “Si se le administra más Ropsine del que debe” anteriormente). Estos incluyen ataques (convulsiones, crisis), sensación de mareo o aturdimiento, entumecimiento de los labios y alrededor de la boca, entumecimiento de la lengua, problemas de audición, problemas con la vista (visión), problemas con el habla (disartria), rigidez de los músculos y temblor, disminución del sentido del tacto (hipoestesia).
- Desmayo (síncope).
- Dificultad al respirar (disnea).
- Temperatura del cuerpo baja.
Raros(puede afectar hasta 1 de cada 1000 personas)
- Ataque al corazón, latido irregular del corazón (arritmias)
Posibles efectos adversos observados con otros anestésicos locales que podrían también ser producidos porRopsineincluyen:
- Entumecimiento, debido a la irritación de los nervios producida por la aguja o por la inyección. Esto, normalmente, no dura mucho.
- Daño en los nervios. Raramente, puede producir problemas permanentes.
- Si se administra demasiada Ropsine en el líquido espinal, puede adormecer todo el cuerpo (anestesiado).
Efectos adversos adicionales en niños
En niños, los efectos adversos son los mismos que en adultos a excepción de la tensión arterial baja, que es menos frecuente en niños (afectan a menos de 1 de cada 10 niños) y sensación de malestar, que son más frecuentes en niños (afectan a más de 1 de cada 10 niños).
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ropsine
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice Ropsine después de la fecha de caducidad que aparece en la ampolla o caja. La fecha de caducidad es el último día del mes que se indica.
No congelar.
No utilice Ropsine si observa alguna precipitación en la solución para inyección.
Normalmente, su médico o el hospital conservarán Ropsine y son responsables de la calidad del producto si una vez abierto no se utiliza inmediatamente. También son responsables de desechar correctamente toda la Ropsine no utilizada.
Los medicamentos no se deben tirar por los desagües, ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deRopsine
- El principio activo es hidrocloruro de ropivacaína 7,5 mg/ml. Cada ampolla de polipropileno de 10 ml contiene 75 mg de ropivacaína (como hidrocloruro).
Cada ampolla de polipropileno de 20 ml contiene 150 mg de ropivacaína (como hidrocloruro).
- Los demás componentes son cloruro de sodio, hidróxido de sodio (para el ajuste de pH) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Ropsine solución inyectable es una solución inyectable acuosa transparente, incolora, estéril, isotónica, isobárica.
Ropsine 7,5 mg/ml solución inyectable EFG está disponible en ampollas de 10 ml y 20 ml transparentes de polipropileno.
Tamaño de envase:
10 ampollas estériles en blíster de plástico.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Sintetica GmbH
Albersloher Weg 11
48155 Münster
Alemania
Responsable de la fabricación:
Sintetica GmbH
Albersloher Weg 11
48155 Münster
Alemania
Fecha de la última revisión de este prospecto:Septiembre 2023.
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
-----------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a médicos o profesionales del sector sanitario:
Manipulación
Ropsine debe ser usado por, o bajo la supervisión de, médicos experimentados en anestesia regional (ver sección 3)
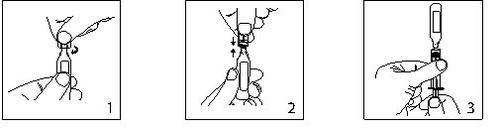
- Mantener la ampolla en posición vertical y girar el cuello para eliminar cualquier resto de solución.
Abrir torciendo bruscamente la parte superior de la ampolla.
- La ampolla puede ser conectada directamente a la jeringa como se muestra en la fig.2.
Las ampollas se ajustan tanto a las jeringas Luerfit como las LuerLock.
- Sostener la jeringa con la ampolla hacia arriba. Sin apretar la ampolla, retirar la solución. Mantener la presión hacia abajo en el émbolo de la jeringa una vez la solución se haya retirado y hasta que la ampolla vacía sea descartada.
Periodo de validez previa apertura
3 años
Periodo de validez después de la apertura
Desde el punto de vista microbiológico, el producto debe ser empleado de forma inmediata. Si no se utiliza inmediatamente, la conservación durante la utilización y las condiciones antes de su empleo son responsabilidad del usuario y generalmente no deberían sobrepasar las 24 horas a 2-8ºC.
Los medicamentos Ropsine son productos sin conservantes y están destinados para un solo uso. Desechar cualquier solución no utilizada.
El medicamento deberá ser inspeccionado visualmente antes de su uso. La solución debe utilizarse únicamente si la solución es transparente, prácticamente libre de partículas y si el envase está intacto.
El envase intacto no se debe re-introducir en el autoclave.
Posología
Adultos y adolescentes mayores de 12 años de edad
La tabla que se muestra a continuación es una guía sobre las dosis más habitualmente empleadas en los diferentes tipos de bloqueo. Deberá emplearse la dosis más pequeña requerida para producir un bloqueo eficaz. La experiencia clínica y el conocimiento de la condición clínica del paciente son factores importantes a la hora de decidir la dosis.
Indicación | Concentración mg/ml | Volumen ml | Dosis mg | Inicio acción minutos | Duración horas |
Anestesia en cirugía | |||||
Administración epidural lumbar | |||||
Cirugía | 7,5 | 15-25 | 113-188 | 10-20 | 3-5 |
10,0 | 15-20 | 150-200 | 10-20 | 4-6 | |
Cesárea | 7,5 | 15-20 | 113-1501) | 10-20 | 3-5 |
Administración epidural torácica | |||||
Establecer un bloqueo para el alivio del dolor en el post-operatorio | 7,5 | 5-15 (dependiendo del nivel de la inyección) | 38-113 | 10-20 | -- |
Bloqueo de troncos nerviosos* | |||||
Bloqueo plexo branquial | 7,5 | 30-40 | 225-3002) | 10-25 | 6-10 |
Bloqueo periférico | |||||
Por ej. bloqueo de nervios menores e infiltración | 7,5 | 1-30 | 7,5-225 | 1-15 | 2-6 |
Las dosis expuestas en la tabla son las consideradas necesarias para producir un bloqueo adecuado y deberán considerarse como recomendaciones de uso en adultos. Tienen lugar variaciones individuales en el inicio y duración de la acción. Las cifras de la columna “Dosis” reflejan el intervalo de dosis promedio necesarias esperado. Se consultará bibliografía adecuada para los factores que afectan a las técnicas de bloqueo específicas y a los requerimientos de cada uno de los pacientes. |
- Con respecto al bloqueo de troncos nerviosos, únicamente puede darse una recomendación posológica para el plexo branquial. Para otros bloqueos del tronco nervioso, pueden requerirse dosis menores. Sin embargo, actualmente no existe experiencia para recomendaciones de dosis específicas para otros bloqueos.
- Deberá administrarse la dosis de forma creciente. La dosis inicial de unos 100 mg (97,5 mg = 13 ml; 105 mg = 14 ml) se administrará durante 3-5 minutos y en caso necesario pueden administrase dos dosis extra, hasta un total de 50 mg adicionales.
- La dosis empleada para el bloqueo de troncos nerviosos debe ajustarse según el lugar de administración y el estado del paciente. Los bloqueos interescaleno y del plexo branquial supraclavicular pueden estar asociados a una mayor frecuencia de reacciones adversas graves, independientemente del anestésico local utilizado (ver sección 4.4).
Generalmente, la anestesia en cirugía (por ejemplo administración epidural) requiere el uso de concentraciones y dosis más altas. Para los procesos quirúrgicos en los cuales es necesario un bloqueo motor profundo, se recomienda la anestesia epidural empleando la formulación de Ropsine 10 mg/ml. Para la analgesia (por ejemplo, la administración epidural para el tratamiento del dolor agudo) se aconsejan las concentraciones y dosis más bajas (2 mg/ml).
Forma de administración
Administración perineural y epidural por inyección.
Antes y durante la inyección se recomienda realizar una aspiración cuidadosa para prevenir una inyección intravascular. Cuando se va a inyectar una dosis más alta, se aconseja una dosis de prueba 3-5 ml de lidocaína 2% (lignocaína) con adrenalina (epinefrina) 1:200.000. Una inyección intravascular accidental puede reconocerse por un incremento temporal en la frecuencia cardíaca y una inyección intratecal accidental por signos de bloqueo espinal.
Se realizará una aspiración antes y durante la administración de la dosis principal, que se inyectará de forma lenta o en dosis crecientes, a una velocidad de 25-50 mg/minuto, mientras se vigilan constantemente las funciones vitales del paciente y se mantiene el contacto verbal con él. Si aparecen síntomas tóxicos, la administración del fármaco deberá interrumpirse inmediatamente.
En el bloqueo epidural para cirugía, se han empleado dosis únicas de hasta 250 mg de hidrocloruro de ropivacaína que fueron bien toleradas.
En el bloqueo del plexo braquial en un número limitado de pacientes se ha utilizado una dosis única de 300 mg que ha resultado ser bien tolerada.
Cuando se requieren bloqueos prolongados, mediante una perfusión continua o la administración en bolo repetida, deberá tenerse en cuenta los riesgos de alcanzar una concentración plasmática tóxica o la posibilidad de inducir lesión neural local. Dosis acumuladas de hasta 675 mg de hidrocloruro de ropivacaína para cirugía y analgesia post-operatoria administradas durante 24 horas fueron bien toleradas en adultos, así como las perfusiones epidurales continuas post-operatorias a velocidades de hasta 28 mg/hora durante 72 horas. En un número limitado de pacientes se han administrado dosis superiores de hasta 800 mg/día con relativamente pocas reacciones adversas.
Para el tratamiento del dolor post-operatorio, se recomienda la siguiente técnica: A no ser que se inicie el tratamiento con Ropivacaína previamente a la intervención, se induce un bloqueo epidural con ésta a una concentración de 7,5 mg/ml empleando un catéter epidural. La analgesia se mantiene con una perfusión de Ropsine 2 mg/ml. Velocidades de perfusión de 6-14 ml (12-28 mg) por hora proporcionan una analgesia adecuada con sólo un ligero y no progresivo bloqueo motor en la mayoría de los casos con dolor post-operatorio y carácter de moderado a severo. La duración máxima del bloqueo epidural es de 3 días. Sin embargo, deberá realizarse un seguimiento estrecho del efecto analgésico con el fin de extraer el catéter tan pronto como el dolor lo permita. Con esta técnica se ha observado una reducción significativa de la necesidad de utilizar opiáceos.
En estudios clínicos, se ha administrado una perfusión epidural de 2 mg/ml de hidrocloruro de ropivacaína sola o mezclada con 1-4 μg/ml de fentanilo para el tratamiento del dolor post-operatorio durante un periodo de hasta 72 horas. Esta combinación de hidrocloruro de ropivacaína y fentanilo proporcionó un mejor alivio del dolor pero causó efectos secundarios de tipo opiáceos; se ha investigado dicha combinación sólo para hidrocloruro de ropivacaína 2 mg/ml.
Cuando se aplican bloqueos nerviosos periféricos prolongados, bien a través de una perfusión continua o mediante inyecciones repetidas, se deben considerar los riesgos de alcanzar una concentración plasmática tóxica o de inducir lesión neural local. En estudios clínicos, se estableció un bloqueo nervioso femoral con 300 mg de hidrocloruro de ropivacaína 7,5 mg/ml y un bloqueo interescaleno con 225 mg de hidrocloruro de ropivacaína 7,5 mg/ml, respectivamente, antes de la cirugía; manteniéndose entonces la analgesia con hidrocloruro de ropivacaína 2 mg/ml. Velocidades de perfusión o inyecciones intermitentes de 10-20 mg por hora durante 48 horas, provocaron una analgesia adecuada y fueron correctamente toleradas.
Concentraciones por encima de 7,5 mg/ml de hidrocloruro de ropivacaína no han sido estudiadas en las intervenciones de cesárea.
Población pediátrica de 0hasta 12 años de edad inclusive
El uso de Ropsine 7,5 mg/ml puede estar asociada con eventos tóxicos sistémicos y centrales en los niños. Concentraciones menores (2 mg/ml, 5mg/ml) son más adecuadas para la administración en esta población.
El empleo de hidrocloruro de ropivacaína en neonatos prematuros no se ha estudiado, en ninguna de las formas de administración.
Forma de administración
Administración epidural por inyección.
Se recomienda una aspiración cuidadosa antes y durante la inyección para prevenir la inyección intravascular. Se observarán estrechamente las funciones vitales del paciente durante la inyección. Si se producen síntomas tóxicos, la inyección deberá interrumpirse inmediatamente.
Una inyección epidural caudal única de 2 mg/ml de hidrocloruro de ropivacaína produce una analgesia post-quirúrgica idónea por debajo de T12 en la mayoría de los pacientes cuando se emplea una dosis de 2 mg/kg en un volumen de 1 ml/kg. Se puede ajustar el volumen de la inyección epidural caudal para obtener una distribución diferente del bloqueo sensorial, tal como se recomienda en la bibliografía. Se han estudiado dosis de hasta 3 mg/kg de una concentración de ropivacaína de 3 mg/ml en niños mayores de 4 años; sin embargo, esta concentración se asocia a una mayor incidencia de bloqueo motor.
Se recomienda fraccionar la dosis de anestésico local calculada, independientemente de la vía de administración.
En caso que se recomiende la infusión de hidrocloruro de ropivacaína, puede utilizarse Ropsine solución inyectable.
Incompatibilidades
No se han investigado compatibilidades con otras soluciones, por lo que este medicamento no debe mezclarse con otros medicamentos.
Se puede producir precipitación en soluciones alcalinas ya que el hidrocloruro de ropivacaína muestra escasa solubilidad a pH > 6,0.
Eliminación
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él, se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ROPSINE 7.5 MG/ML SOLUCION INYECTABLE EFGForma farmacéutica: INYECTABLE, 100 MGPrincipio activo: ropivacaineFabricante: Altan Pharmaceuticals SaRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 2 mg/mlPrincipio activo: ropivacaineFabricante: Altan Pharmaceuticals SaRequiere recetaForma farmacéutica: INYECTABLE, 75 MGPrincipio activo: ropivacaineFabricante: Altan Pharmaceuticals SaRequiere receta
Médicos online para ROPSINE 7.5 MG/ML SOLUCION INYECTABLE EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ROPSINE 7.5 MG/ML SOLUCION INYECTABLE EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















