
ROLUFTA ELLIPTA 55 MICROGRAMOS POLVO PARA INHALACION (UNIDOSIS)

Cómo usar ROLUFTA ELLIPTA 55 MICROGRAMOS POLVO PARA INHALACION (UNIDOSIS)
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
RoluftaEllipta55microgramospolvo para inhalación (unidosis)umeclidinio (umeclidinium)
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Rolufta Ellipta y para qué se utiliza
- Qué necesita saber antes de empezar a usar Rolufta Ellipta
- Cómo usar Rolufta Ellipta
- Posibles efectos adversos
- Conservación de Rolufta Ellipta
- Contenido del envase e información adicional
Instrucciones de uso paso a paso
1. Qué es Rolufta Ellipta y para qué se utiliza
Qué es Rolufta Ellipta
Rolufta Ellipta contiene el principio activo umeclidinio (como bromuro), que pertenece a un grupo de medicamentos denominados broncodilatadores.
Para qué se utiliza RoluftaEllipta
Este medicamento se utiliza para tratar la enfermedad pulmonar obstructiva crónica(EPOC)en adultos. La EPOC es una enfermedad crónica que empeora lentamente, y en la que, de forma gradual, las vías aéreas y los sacos de aire de los pulmones, se obstruyen o se dañan, lo que provoca dificultad para respirar. Esta dificultad para respirar se añade a la contracción de los músculos que rodean las vías aéreas, lo que hace que estas vías se estrechen y dificulten el flujo del aire.
Este medicamento impide la contracción de estos músculos en los pulmones, facilitando la entrada y salida de aire de los pulmones. Cuando se utiliza de forma regular, ayuda a controlar las dificultades para respirar y reduce los efectos de la EPOC en su vida cotidiana.
RoluftaElliptano se debe utilizar para aliviar un ataque repentino de ahogo o sibilancias (sonidos silbantes al respirar).
Si tiene este tipo de ataques debe utilizar un inhalador de “rescate” de acción rápida (como salbutamol). Si no tiene un inhalador de acción rápida contacte con su médico.
2. Qué necesita saber antes de empezar a usar Rolufta Ellipta
No use RoluftaEllipta
- si es alérgicoa umeclidinio o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Si piensa que lo anterior le aplica, no useeste medicamento hasta haber consultado con su médico.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Rolufta Ellipta:
- si tiene asma(no use Rolufta Ellipta para tratar el asma)
- si tiene problemas cardiacos
- si tiene un problema ocular llamado glaucoma de ángulo cerrado
- si tiene próstata agrandada, dificultad para orinaro una obstrucción en la vejiga
- si tiene problemas graves de hígado.
Consulte con su médicosi piensa que cualquiera de las condiciones anteriores le aplican.
Dificultades respiratorias inmediatasSi tiene opresión en el pecho, tos, sibilancias o dificultad para respirar inmediatamente después de utilizar su inhalador Rolufta Ellipta:
deje de usar este medicamentoy busque atención médica inmediatamente, ya que puede tener una afección grave llamada broncoespasmo paradójico.
Problemas oculares durante el tratamiento con RoluftaEllipta
Si tiene dolor ocular o molestias, visión borrosa durante un tiempo, halos visuales o imágenes coloreadas asociadas a enrojecimiento de los ojos durante el tratamiento con Rolufta Ellipta, deje de usar este medicamento y busque ayuda médica inmediatamenteya que estos signos pueden deberse a un ataque agudo de glaucoma de ángulo cerrado.
Niños y adolescentes
No administre este medicamento a niños o adolescentes menores de 18 años.
Otros medicamentos y RoluftaEllipta
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Si no está seguro de qué contiene su medicamento, consulte con su médico o farmacéutico.
En particular, informe a su médico o farmacéutico si está tomando otros medicamentos de acción prolongada para tratar problemas respiratorios similares a este medicamento, por ejemplo, tiotropio. No utilice Rolufta Ellipta junto con estos medicamentos.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médicoantes de utilizar este medicamento. Si está embarazada, no utilice este medicamento a menos que su médico se lo indique.
Se desconoce si los componentes de Rolufta Ellipta pueden excretarse en la leche materna. Si está en periodo de lactancia, debe consultar con su médicoantes de utilizar Rolufta Ellipta.
Si está en periodo de lactancia, no utilice este medicamento a menos que su médico se lo indique.
Conducción y uso de máquinas
Es poco probable que este medicamento afecte a su capacidad para conducir o utilizar máquinas.
RoluftaElliptacontiene lactosa
Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de utilizar este medicamento.
3. Cómo usar Rolufta Ellipta
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendadaes una inhalación todos los días, a la misma hora cada día. Solo necesita una inhalación al día, ya que el efecto de este medicamento dura 24 horas.
No utilice más dosis de las que su médico le haya indicado.
Use RoluftaElliptaconregularidad
Es muy importante que utilice Rolufta Ellipta todos los días, como le haya indicado su médico. Esto le ayudará a no tener síntomas a lo largo del día y la noche.
Noutilice este medicamento para aliviar un ataque repentino de ahogo o sibilancias. Si tiene este tipo de ataque debe utilizar un inhalador de “rescate” de acción rápida (como salbutamol).
Cómo usar el inhalador
Para obtener la información completa lea las “Instrucciones de uso paso a paso” al final de este prospecto.
La administración de Rolufta Ellipta es por vía inhalatoria. Para usar Rolufta Ellipta, inspírelo hacia sus pulmones a través de la boca utilizando el inhalador Ellipta.
Si los síntomas no mejoran
Si sus síntomas de EPOC (ahogo, sibilancias, tos) no mejoran o empeoran, o si está utilizando su inhalador de “rescate” de acción rápida más a menudo de lo habitual:
contacte con su
médico lo antes posible.
Si usa más RoluftaElliptadel que debe
Si accidentalmente usa demasiado medicamento, contacte con su médico o farmacéutico inmediatamente,ya que puede necesitar atención médica. Si es posible, muéstreles el inhalador, el envase o su prospecto. Podría notar que su corazón late más rápido de lo normal, tener alteraciones visuales o la boca seca.
Si olvidó usar RoluftaEllipta
No inhale una dosis doble para compensar las dosis olvidadas.Inhale la siguiente dosis a su hora habitual.
Si tiene sibilancias o ahogo, utilice su inhalador de “rescate” de acción rápida (por ejemplo salbutamol), y busque asesoramiento médico.
Si interrumpe el tratamiento con RoluftaEllipta
Utilice este medicamento durante el tiempo que le haya recomendado su médico. Solo será eficaz durante el tiempo que siga utilizándolo. No deje de utilizarlo hasta que su médico se lo indique, aunque se encuentre mejor, ya que sus síntomas pueden empeorar.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones alérgicas
Si después de usar Rolufta Ellipta tiene cualquiera de los siguientes síntomas, deje de usar este medicamento e informe inmediatamente a su médico:
Poco frecuentes(pueden afectar hasta 1 de cada 100personas):
- picazón
- erupción cutánea (habones) o enrojecimiento.
Raros(pueden afectar hasta 1 de cada 1000personas):
- sibilancias (sonido silbante que se produce al respirar), tos o tener dificultad para respirar
- sensación de debilidad repentina o mareo (que puede provocar colapso o pérdida de la conciencia)
Otros efectos adversos:
Frecuentes(pueden afectar hasta1 de cada 10personas):
- latido del corazón más rápido
- dolor al orinar y aumento de la frecuencia (pueden ser signos de una infección del tracto urinario)
- resfriado común
- infección de la nariz y garganta
- tos
- sensación de presión o dolor en las mejillas y en la frente (pueden ser síntomas de inflamación de los senos nasales llamada sinusitis)
- dolor de cabeza
- estreñimiento
- dolor de boca y de garganta.
Poco frecuentes(pueden afectar hasta 1 de cada 100personas):
- latido del corazón irregular
- dolor de garganta
- boca seca
- alteración del gusto
- ronquera.
Raros(pueden afectar hasta 1 de cada 1.000personas):
- dolor ocular.
Frecuencia no conocida(la frecuencia no se puede estimar a partir de los datos disponibles):
- disminución en la visión o dolor en los ojos debido a una presión ocular elevada (posibles signos de glaucoma)
- visión borrosa
- aumento de la presión ocular
- dificultad y dolor durante el paso de la orina, esto puede ser signo de obstrucción de la vejiga o retención urinaria
- mareo.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Rolufta Ellipta
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase, bandeja e inhalador, después de CAD. La fecha de caducidad es el último día del mes que se indica.
Mantener el inhalador dentro de la bandeja sellada para protegerlo de la humedad y solo sacarlo inmediatamente antes del primer uso. Una vez abierta la bandeja, el inhalador puede utilizarse durante un plazo de 6 semanas, contando desde la fecha de apertura de la bandeja. Escriba la fecha en la que se debe tirar el inhalador en el espacio designado para ello en la etiqueta del inhalador. La fecha se debe anotar tan pronto como el inhalador se saque de la bandeja.
No conservar a temperatura superior a 30 °C.
Si lo conserva en la nevera, deje que el inhalador vuelva a la temperatura ambiente al menos una hora antes de utilizarlo.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de RoluftaEllipta
El principio activo es umeclidinio (como bromuro).
Cada inhalación proporciona una dosis liberada (dosis que sale por la boquilla) de 55 microgramos de umeclidinio (equivalente a 65 microgramos de bromuro de umeclidinio).
Los demás componentes son lactosa monohidrato (ver apartado “Rolufta Ellipta contiene lactosa” en la sección 2) y estearato de magnesio.
Aspecto del producto y contenido del envase
Rolufta Ellipta es un polvo para inhalación (unidosis).
El inhalador Ellipta está formado por un cuerpo de plástico gris, un protector de la boquilla verde claro y un contador de dosis. Está envasado en una bandeja de aluminio laminado con una lámina de aluminio desplegable. La bandeja contiene una bolsa con desecante para reducir la humedad en el envase.
El principio activo se presenta como un polvo blanco en un blíster dentro del inhalador.
Rolufta Ellipta está disponible en envases de un inhalador que contienen 7 o 30 dosis y en envases múltiples de 90 dosis (3 inhaladores de 30 dosis). Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización:
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublín 24
Irlanda
Responsable de la fabricación:
Glaxo Wellcome Production
Zone Industrielle No.2
23 Rue Lavoisier
27000 Evreux
Francia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals s.a./n.v. Tél/Tel: + 32 (0) 10 85 52 00 | Lietuva UAB “BERLIN-CHEMIE MENARINI BALTIC” Tel: +370 52 691 947 | ||
| Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals s.a./n.v. Belgique/Belgien Tél/Tel: + 32 (0) 10 85 52 00 | ||
Ceská republika GlaxoSmithKline, s.r.o. Tel: + 420 222 001 111 | Magyarország Berlin-Chemie/A. Menarini Kft. Tel.:+36 23501301 | ||
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Trading Services Limited Tel: +356 80065004 | ||
Deutschland BERLIN-CHEMIE AG Tel: +49 (0) 30 67070 | Nederland GlaxoSmithKline BV Tel: + 31 (0) 33 208110030 | ||
Eesti OÜ Berlin-Chemie Menarini Eesti Tel: +372 667 5001 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 | ||
Ελλ?δα GlaxoSmithKline Μονοπρ?σωπη A.E.B.E. Τηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 | ||
España FAES FARMA, S.A. Tel.: +34 900 460 153 | Polska GSK Services Sp. z o.o. Tel.: + 48 (0)22 576 9000 | ||
France Laboratoire GlaxoSmithKline Tél: + 33 (0)1 39 17 84 44 | Portugal GlaxoSmithKline – Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 | ||
Hrvatska Berlin-Chemie Menarini Hrvatska d.o.o. Tel: +385 1 4821 361 | România GlaxoSmithKline Trading Services Limited Tel: +40 800672524 | ||
Ireland GlaxoSmithKline (Ireland) Limited Tel: + 353 (0)1 4955000 | Slovenija Berlin-Chemie / A. Menarini Distribution Ljubljana d.o.o. Tel: +386 (0)1 300 2160 | ||
Ísland Vistor hf. Sími: + 354 535 7000 | Slovenská republika Berlin-Chemie / A. Menarini Distribution Slovakia s.r.o. Tel: +421 2 544 30 730 | ||
Italia
Tel: +39-055 56801 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 (0)10 30 30 30 | ||
Κ?προς GlaxoSmithKline Trading Services Limited Τηλ: +357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 | ||
Latvija SIA Berlin-Chemie/Menarini Baltic Tel: +371 67103210 | United Kingdom (Northern Ireland) GlaxoSmithKline Trading Services Limited Tel: + 44 (0)800 221441 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Instrucciones de uso paso a paso
¿Qué es el inhalador Ellipta?
La primera vez que utilice Rolufta Ellipta, no necesita verificar que el inhalador esté funcionando correctamente, ya que contiene dosis previamente medidas y está listo para utilizarse directamente.
Su caja de inhalador Rolufta Ellipta contiene:
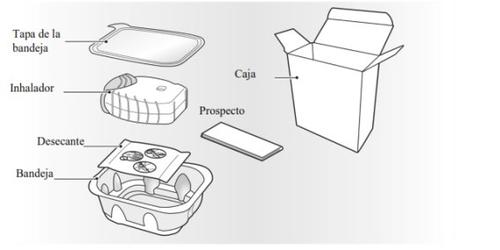
El inhalador está envasado en una bandeja. No abra la bandeja hasta que esté preparado para empezar a usar su inhalador nuevo. Cuando esté preparado para usar el inhalador, retire la tapa para abrir la bandeja. La bandeja contiene una bolsa desecante, para reducir la humedad. Tire la bolsa del desecante, nola abra, ingiera o inhale.
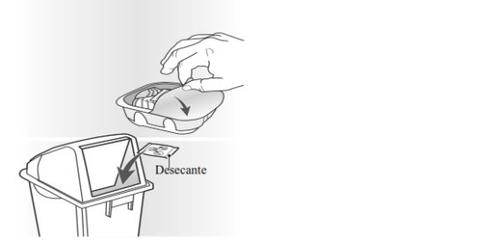
Cuando saque el inhalador de su bandeja, estará en la posición de “cerrado”. No abra el inhalador hasta que esté preparado para inhalar una dosis del medicamento. Cuando se abre la bandeja, se debe anotar la fecha de “Desechar el” en el espacio designado para ello que aparece en la etiqueta del inhalador. La fecha de “desechar el” es de 6 semanas desde la fecha de apertura de la bandeja. Después de esta fecha el inhalador no debe utilizarse más. La bandeja se puede desechar una vez que lo abra.
Si se conserva en nevera, deje que el inhalador alcance la temperatura ambiente durante al menos una hora antes de su uso.
Las instrucciones de uso paso a paso del inhalador Ellipta proporcionadas a continuación pueden ser usadas tanto para el inhalador de 30 dosis (30 días de tratamiento) como para el inhalador de 7 dosis (7 días de tratamiento).
- Lea esta información antes de comenzar
Si la tapa del inhalador se abre y se cierra sin que se inhale el medicamento, se perderá la dosis.La dosis perdida quedará retenida de forma segura dentro del inhalador, pero no estará disponible para ser inhalada.
No es posible administrar de forma accidental una dosis adicional o una dosis doble mediante una inhalación.
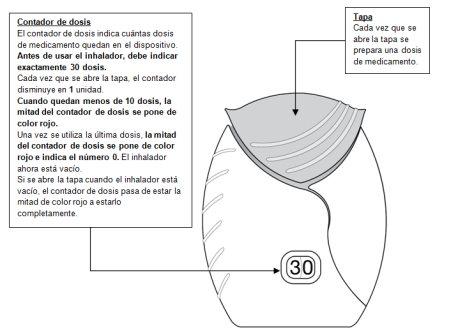
- Preparar una dosis
Espere a abrir la tapa del inhalador hasta que estépreparado para inhalar una dosis.
No agite el inhalador.
- Deslizar la tapa hacia abajo hasta oír un ‘clic’.
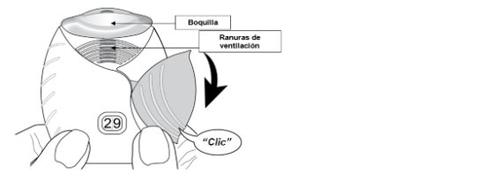
Ahora, el medicamento está preparado para poder inhalarlo.
Como confirmación, el contador de dosis disminuye en 1unidad.
- Si el contador de dosis no disminuye al oír el ‘clic’, el inhalador no liberará la dosis del medicamento.
Llévelo al farmacéutico y solicite ayuda.
- Inhale su medicamento
- Mientras mantiene el inhalador alejado de la boca, espire tanto como le sea posible.
Noespire dentro del inhalador.
- Coloque la boquilla entre los labios, y ciérrelos firmemente alrededor de la boquilla.
Nobloquee las ranuras de ventilación con los dedos.
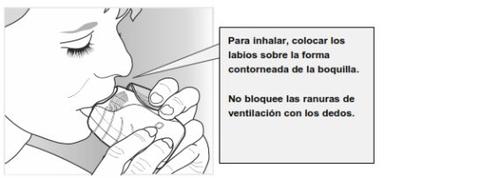
- Realice una inspiración larga, continua y profunda. Mantenga la inspiración tanto tiempo como sea posible (al menos 3-4 segundos).
- Retire el inhalador de la boca.
- Espire suave y lentamente.
Puede que no sea capaz de distinguir el sabor o notar el medicamento, incluso cuando utiliza el inhalador de forma correcta.
Antesde cerrar la tapa, la boquilla del inhalador puede limpiarse utilizando un pañuelo seco.
- Cerrar el inhalador
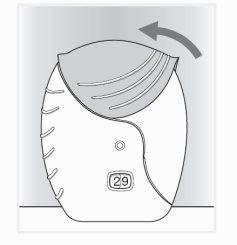
- Deslice la tapa hacia arriba, hasta el tope, para cubrir la boquilla.
- País de registro
- Precio medio en farmacia45.27 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ROLUFTA ELLIPTA 55 MICROGRAMOS POLVO PARA INHALACION (UNIDOSIS)Forma farmacéutica: INHALACIÓN PULMONAR, 55 microgramosPrincipio activo: umeclidinium bromideFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 0,0040 g/aerosolPrincipio activo: Ipratropio bromuroFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 20 µgPrincipio activo: Ipratropio bromuroFabricante: Boehringer Ingelheim Espana S.A.Requiere receta
Médicos online para ROLUFTA ELLIPTA 55 MICROGRAMOS POLVO PARA INHALACION (UNIDOSIS)
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ROLUFTA ELLIPTA 55 MICROGRAMOS POLVO PARA INHALACION (UNIDOSIS), sujeto a valoración médica y a la normativa local.
Preguntas frecuentes
















