
RHINOCORT 64 microgramos SUSPENSION PARA PULVERIZACION NASAL

Cómo usar RHINOCORT 64 microgramos SUSPENSION PARA PULVERIZACION NASAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Rhinocort 64 microgramos suspensión para pulverización nasal
Budesonida
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted. Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico, o farmacéutico.
|
Contenido del prospecto
- Qué es Rhinocort 64 microgramos y para qué se utiliza.
- Qué necesita saber antes de empezar a usar Rhinocort 64 microgramos.
- Cómo usar Rhinocort 64 microgramos.
- Posibles efectos adversos.
- Conservación de Rhinocort 64 microgramos.
- Contenido del envase e información adicional.
1. Qué es Rhinocort 64 microgramos y para qué se utiliza
Rhinocort 64 microgramos contiene como principio activo budesonida, que pertenece a un grupo de medicamentos llamados glucocorticoides y se emplea para reducir la inflamación. Rhinocort 64 microgramos reduce la inflamación de la mucosa nasal (parte interna de la nariz).
Rhinocort 64 microgramos se utiliza para el alivio de los síntomas nasales (tales como estornudos, picor, goteo nasal, congestión) de la rinitis alérgica producida por el polen y otros alérgenos presentes en el aire, tales como ácaros del polvo, esporas de hongos o pelo de animales, en adultos.
El máximo nivel de protección puede no alcanzarse durante los primeros días, por lo que es importante continuar un uso regular para garantizar el beneficio terapéutico completo.
Debe consultarse al médico si los síntomas no mejoran o empeoran después de 7 días.
Este medicamento no debe utilizarse de forma continuada durante más de 3 meses, a no ser que su médico se lo indique.
2. Qué necesita saber antes de empezar a usar Rhinocort 64 microgramos
No use Rhinocort 64 microgramos:
- Si es alérgico al principio activo o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si presenta síntomas o signos de una infección localizada en las fosas nasales.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Rhinocort 64 microgramos si:
- Sufre hemorragias nasalesgraves o frecuentes
- Si ha sufrido úlceras nasales recientes, se ha sometido a una intervención quirúrgica nasalo ha sufrido una herida nasalque no se ha curado bien.
- Sufre tuberculosis, una infección pectoral, varicela o sarampióno ha estado en contacto con alguien que sufre tuberculosis, varicela o sarampión.
- Ha sufrido alguna vez glaucoma(aumento de la presión del ojo) o cataratas.
- Padece una infección ocular.
- Tiene diabetes.
- Tiene problemas de hígado.
Consulte a su médico o farmacéutico si sufre cualquiera de los trastornos siguientes mientras usa Rhinocort 64 microgramos:
- Visión borrosau otras alteraciones visuales
- Cualquier signo de infección,como fiebre persistente
Si los síntomas persisten o empeoran, o si se presentan nuevos síntomas, deben dejar de utilizar Rhinocort 64 microgramos y consultar a su médico.
Niños y adolescentes
No se conocen los efectos a largo plazo en niños, pero puede ocasionar un retraso del crecimiento. Si se observa que se enlentece el crecimiento se debe consultar al médico para valorar el tratamiento.
Uso de Rhinocort 64 microgramos con otros medicamentos:
Informe a su médico o farmacéutico si está usando, ha usado recientemente o podría tener que utilizar cualquier otro medicamento. Esto incluye los medicamentos adquiridos sin receta médica y las plantas medicinales. Algunos medicamentos pueden aumentar los efectos de Rhinocort. En particular, informe a su médico o farmacéutico si está tomando los sigueintes medicamentos:
- Para tratar infecciones por hongos(como itraconazol o ketoconazol).
- Está tomando antibióticos (como claritromicina)
- Está tomando medicamentos para el VIH (como saquinavir, atazanavir, indinavir, nelfinavir, ritonavir o medicamentos que contienen cobicistat).
- Está tomando cimetidina (medicamento para la acidez del estómago).
- Está tomando estrógenoscomo terapia hormonal sustitutivao píldoras anticonceptivas.
- Está tomando otro medicamento con corticosteroides(como cremas para el eccema, inhaladores para el asma, comprimidos, inyecciones, pulverizadores nasales, gotas para los ojos o la nariz).
- Acaba de interrumpir el uso de comprimidos con corticosteroidescomo prednisolonao inyecciones de corticosteroides.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.. Debe contactar con su médico lo antes posible si se queda embarazada durante el tratamiento con Rhinocort 64 microgramos.
Conducción y uso de máquinas
No hay indicios de que Rhinocort 64 microgramos pueda afectar su capacidad de conducción o manejo de maquinaria.
Rhinocort 64 microgramos contiene sorbato de potasio.
Dado que Rhinocort 64 microgramos contiene sorbato de potasio como excipiente, puede provocar reacciones cutáneas locales (p.ej., dermatitis de contacto)
Advertencia a los deportistas:Se informa a los deportistas que este medicamento contiene un componente que puede producir un resultado positivo en las pruebas de control de dopaje.
3. Cómo usar Rhinocort 64 microgramos
Siga exactamente las instrucciones de administración de Rhinocort 64 microgramos contenidas en este prospecto o lasindicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Adultos:
La dosis recomendada diaria inicial son dos pulverizaciones en cada orificio nasal.
Esta dosis puede administrarse como dos aplicaciones (2 x 64 microgramos) en cada fosa nasal una vez al día por la mañana o una aplicación (1 x 64 microgramos) en cada fosa nasal, por la mañana y por la noche.
Una vez alcanzado el alivio total de los síntomas, se puede reducir la administración a una aplicación en cada orificio nasal una vez al día, preferiblemente por la mañana.
No administre más de cuatro aplicaciones en un día.
Debe consultar a un médico o farmacéutico si empeora o no mejora después de 7 días.
No lo use de forma continuada durante más de 3 meses.
Es posible que el note alivio de los síntomas desde el primer día de tratamiento, sin embargo, pueden ser necesarios varios días de tratamiento para que se produzca el efecto completo (en ocasiones hasta 2 semanas).
No debe compartir el pulverizador con otras personas debido al riesgo de contagio.
Debe evitarse el contacto del producto con los ojos. En caso de que se produzca contacto con los ojos, láveselos inmediatamente con agua abundante.
Antes de utilizar Rhinocort 64 microgramos por primera vez, deberá leer el apartado “Forma de administración”,en el que se explica cómo funciona la bomba pulverizadora. Siga las instrucciones cuidadosamente.
Uso en niños
Este medicamento no debe utilizarse en niños ni en adolescentes menores de 18 años.
Si usa más Rhinocort 64 microgramos del que debe
La administración en una única ocasión de más dosis de las recomendadas no suele causar efectos perjudiciales. Si esto ocurre durante un largo periodo de tiempo (meses), es posible la aparición de efectos adversos, por lo que debería consultar a su médico o a su farmacéutico.
En caso de sobredosis o ingestión accidental, acudir inmediatamente a un centro médico o llamar al Servicio de Información Toxicológica, teléfono +34915620420, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Rhinocort 64 microgramos:
En caso de olvido de una dosis de Rhinocort 64 microgramos, espere a la siguiente. No tome una dosis doble para compensar las dosis olvidadas. Simplemente aplique la dosis siguiente como sea necesario.
Forma de administración
Lea atentamente las siguientes instrucciones de uso y sígalas cuidadosamente.
Antes de utilizar el pulverizador nasal de Rhinocort 64 microgramos por primera vez, agite el envase , quite la tapa protectora y, manteniendo el envase en posición vertical, pulse varias veces (5-10 veces) al aire hasta que empiece a aparecer una pulverización uniforme del producto (ver Figura 1). Si no se utiliza diariamente, es necesario cargar de nuevo la bomba. En este caso, será suficiente realizar una (1) sola pulsación al aire.
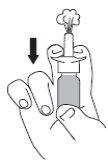
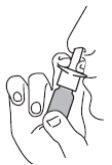
- Suénese la nariz. Agite el envase y quite la tapa.
- Sostenga el envase en posición vertical tal como se muestra en las figuras.
- Introduzca la punta del aplicador en un orificio nasal y diríjalo al lateral de la nariz en la dirección contraria al centro de la nariz (el «tabique»). Pulse una o dos veces, dependiendo de la dosis necesaria.Repita la acción en el otro orificio nasal.
- Vuelva a colocar la tapa . No use Rhinocort 64 microgramos pulverizador nasal más veces de las recomendadas en este prospecto.
Limpieza
Limpie regularmente las piezas superiores de plástico. Para ello, quite la tapa y el aplicador nasal. Lave las piezasde plástico con agua templada. Déjelos secar completamente al aire antes de volverlos a colocar.
No intente limpiar el aplicador nasal con un alfiler ni con ningún objeto punzante.
Cuando ya no necesite este medicamento, es recomendable desechar el resto, aunque no se haya consumido en su totalidad.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si experimenta cualquiera de las siguientes dolencias, deje de usar este pulverizador nasal y consulte inmediatamente a un médico:
- signos repentinos de reacción alérgica como sarpullido, picor, ronchas o enrojecimiento de la piel, hinchazón de la cara, los labios, la boca, la lengua u otras partes del cuerpo, dificultad para respirar, sibilancias o dificultad para tragar o respirar o sensación de desmayo.
Otros efectosadversosincluyen:
Frecuentes (pueden afectar a 1 de cada 10 pacientes):
- Infecciones de la nariz, la garganta o los senos paranasales:
- Hemorragia nasal (epistaxis) o irritación de la nariz.
- Dolor en la boca y/o garganta.
- Infección de oidos
- Dolor de cabeza
- Molestias abdominales
- Fiebre
Poco frecuentes (pueden afectar hasta 1 de cada
100 pacientes):
- Calambres musculares.
Raras (pueden afectar hasta 1 de cada 1.000 pacientes):
- Efectos sobre las glándulas suprarrenales (glándulas de pequeño tamaño situadas junto a los riñones).
- Úlceras nasales o perforación de la membrana que separa los orificios nasales (tabique nasal).
- Cambio de voz
- Hematomas
- Visión borrosa.
Otros efectos adversos de los que no se conoce la frecuencia incluyen:
- Cataratas (pérdida de transparencia del cristalino en el ojo).
- Glaucoma (aumento de la presión ocular).
- Angioedema.
- Dermatitis.
- Eritema.
- Erupción.
- Urticaria.
Otros efectos secundarios en niños
Se ha notificado menor crecimiento en niños tratados con corticosteroides nasales.
En niños también se han notificado frecuentemente los siguientes efectos adversos: molestias estomacales ,dolor de cabeza, tos, fiebre o temperatura alta, inflamación e infecciones de los oídos, amígdalas, senos paranasales o pulmones o erupción cutánea.
Se han notificado en raras ocasiones (especialmente en niños) trastornos mentales o de la conducta, como hiperactividad, trastornos del sueño, nerviosismo, depresión o agresividad.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Rhinocort 64 microgramos
Vuelva a colocar la tapa protectora después de utilizar Rhinocort 64 microgramos.
No conservar a temperatura superior a 30 °C. No congelar.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Rhinocort 64 microgramos
- El principio activo es budesónida.
- Los demás componentes (excipientes) son: edetato de disodio, sorbato de potasio (E 202), glucosa anhidra, celulosa microcristalina (E 460) y carboximetil celulosa sódica (E 466), polisorbato 80 (E 433), ácido clorhídrico y agua purificada.
Aspecto del producto y contenido del envase
Rhinocort 64 microgramos se presenta en un envase de vidrio marrón de 10 ml (120 pulverizaciones) provisto de una bomba pulverizadora y un aplicador nasal. El líquido que contiene es una suspensión destinada a la pulverización nasal. Cada pulverización contiene 64 microgramos del principio activo.
Titular de la autorización de comercialización y responsable de la fabricación
El titular es:
JNTL Consumer Health (Spain), S.L.
C/ Vía de los Poblados 1, Edificio E, planta 3
28033-Madrid
España
El responsable de la fabricación es:
McNeil AB
Norrbroplatsen 2
251 09 Helsingborg
Suecia
Fecha de última revisión de este prospecto: Marzo 2022
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/)
- País de registro
- Principio activo
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a RHINOCORT 64 microgramos SUSPENSION PARA PULVERIZACION NASALForma farmacéutica: PRODUCTO USO NASAL, 64 MCG/pulverizaciónPrincipio activo: BudesonidaFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 2 mg budesonida/ mlPrincipio activo: BudesonidaFabricante: M4 Pharma S.L.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 1 mg budesonisa/mlPrincipio activo: BudesonidaFabricante: M4 Pharma S.L.Requiere receta
Médicos online para RHINOCORT 64 microgramos SUSPENSION PARA PULVERIZACION NASAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de RHINOCORT 64 microgramos SUSPENSION PARA PULVERIZACION NASAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











