
REVOLADE 50 MG COMPRIMIDOS RECUBIERTOS CON PELICULA

Cómo usar REVOLADE 50 MG COMPRIMIDOS RECUBIERTOS CON PELICULA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Revolade 12,5mg comprimidos recubiertos con película
Revolade 25mg comprimidos recubiertos con película
Revolade 50mg comprimidos recubiertos con película
Revolade 75mg comprimidos recubiertos con película
eltrombopag
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Revolade y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Revolade
- Cómo tomar Revolade
- Posibles efectos adversos
- Conservación de Revolade
- Contenido del envase e información adicional
1. Qué es Revolade y para qué se utiliza
Revolade contiene eltrombopag, que pertenece a un grupo de medicamentos llamados agonistas del receptor de trombopoyetina, que se utiliza para ayudar a aumentar el número de plaquetas en sangre. Las plaquetas son células presentes en la sangre que ayudan a reducir o prevenir hemorragias.
- Revolade se utiliza para tratar un trastorno de la sangre llamado trombocitopenia inmune (primaria) (PTI) en pacientes de más de 1 año de edad que ya han tomado otros medicamentos (corticosteroides o inmunoglobulinas) que no les han funcionado.
La PTI está causada por un recuento bajo de plaquetas (trombocitopenia). Las personas con PTI tienen mayor riesgo de tener hemorragias. Los síntomas que los pacientes con PTI pueden notar son petequias (puntitos rojos redondos y planos localizados bajo la piel), cardenales, sangrado de nariz, sangrado de encías e incapacidad para controlar el sangrado si se cortan o se hieren.
- Revolade también se puede utilizar para tratar los niveles bajos de plaquetas (trombocitopenia) en adultos con infección del virus de la hepatitis C (VHC), en el caso de que hubieran tenido problemas con los efectos adversos del tratamiento con interferón. Muchas personas con hepatitis C tienen niveles de plaquetas bajos, no sólo por la enfermedad, sino también debido a los tratamientos antivirales que se utilizan para tratarla. Tomar Revolade puede ayudarle a completar el ciclo con los antivirales (peginterferon y ribavirina).
Revolade también se puede utilizar en pacientes con bajos niveles de células sanguineas provocado por una anemia aplásica grave (AAG). La AAG es una enfermedad en la cual la médula ósea está dañada, provocando una deficiencia de glóbulos rojos (anemia), de glóbulos blancos (leucopenia) y de plaquetas (trombocitopenia).
2. Qué necesita saber antes de empezar a tomar Revolade
No tome Revolade
- si es alérgicoa eltrombopag o a alguno de los demás componentes de este medicamento (incluidos en la sección 6 bajo el título “Composición de Revolade”).
Consulte con su médicosi cree que esto puede afectarle.
Advertencias y precauciones
Consulte a su médico antes de empezar a tomar Revolade:
- si tiene problemas de hígado.Las personas que tienen un recuento de plaquetas bajo así como una enfermedad crónica de hígado avanzada (desde hace mucho tiempo), tienen más riesgo de efectos adversos, de daño en el hígado que puede ser mortal y de coagulos de sangre. Si su médico considera que el beneficio de Revolade supera los riesgos, le tendrá muy controlado durante el tratamiento.
- si tiene riesgo de sufrir un tromboen venas o arterias, o si sabe que la aparición de trombos es algo frecuente en su familia.
El riesgo de sufrir un trombo puede ser mayoren las siguientes circunstancias:
- si tiene edad avanzada
- si ha estado en cama durante un largo periodo de tiempo
- si tiene cáncer
- si está tomando la píldora anticonceptiva o terapia hormonal sustitutiva
- si ha sido sometido a cirugía recientemente o si ha sufrido un daño físico
- si tiene mucho sobrepeso (obesidad)
- si es fumador
- si tiene una enfermedad crónica y avanzada en el hígado.
Si se encuentra en cualquiera de estas situaciones, informe a su médicoantes de iniciar el tratamiento. No debe tomar Revolade a menos que su médico considere que el beneficio esperado supera el riesgo de tener trombos.
- si tiene cataratas(el cristalino, la lente del ojo, se enturbia).
- si tiene otra enfermedad de la sangre, como el síndrome mielodisplásico (SMD). Antes de empezar a tomar Revolade, su médico le realizará pruebas para comprobar que no tiene esta enfermedad. Si tiene SMD y toma Revolade, el SMD puede empeorar.
Informe a su médicosi se encuentra en alguna de estas situaciones.
Exámenes oculares
Su médico le recomendará que se haga una revisión para comprobar si tiene cataratas. Si no se hace revisiones rutinarias de los ojos, su médico le pedirá que las haga. También deben revisarle la retina (cápa de células sensible a la luz que se encuentra en la parte posterior del ojo), para ver si existe sangrado en la retina o alrededor de ella.
Necesitará hacerse análisis de forma regular
Antes de que empiece a tomar Revolade, su médico le hará un análisis de sangre para ver como están sus células sanguíneas, incluyendo las plaquetas. Estos análisis se repetirán con frecuencia mientras esté tomando el medicamento.
Análisis de sangre para comprobar la función del hígado
Revolade puede provocarle resultados del análisis de sangre que indiquen daño en el hígado - un aumento de algunas enzímas hepáticas, especialmente bilirrubina y alanina/aspartato transaminasa. Si está tomando interferón, tratamiento base junto con Revolade para tratar los niveles bajos de plaquetas debidos a la hepatitis C, podrían empeorar alguno de los problemas hepáticos.
Se le realizarán análisis de sangre antes de empezar a tomar Revolade y con frecuencia mientras lo esté tomando para comprobar su función hepática. Puede ser necesario que interrumpa el tratamiento con Revolade si los niveles de estos marcadores aumentan demasiado o si tiene cualquier otro signo de daño hepático.
Lea la información “Problemas de hígado” en la sección 4 de este prospecto
Análisis de sangre para el recuento (niveles) de plaquetas
Si interrumpe el tratamiento con Revolade, es probable que a los pocos días, vuelva a presentar unos niveles de plaquetas bajos (trombocitopenia). Se controlarán los niveles de plaquetas y su médico le indicará cuáles son las precauciones que ha de tomar.
Niveles de plaquetas muy altos, podría aumentar el riesgo de formación de trombos. Sin embargo, los trombos pueden también formarse con niveles de plaquetas normales e incluso bajos. Su médico ajustará la dosis de Revolade para asegurar que el recuento de plaquetas no llegue a ser demasiado alto.
Busque ayuda médica inmediatamentesi presenta cualquiera de estos signos de aparición de un trombo:
- hinchazón, doloro sensibilidad en una pierna
- dificultad respiratoria repentina, excepcionalmente acompañada de dolor agudo en el pecho o respiración rápida
- dolor abdominal(estómago), abdomen agrandado, sangre en las heces.
Análisis para examinar su médula ósea
En personas con alteraciones en la médula ósea, medicamentos como Revolade pueden empeorar las alteraciones. Los signos de cambios en la médula ósea pueden aparecer como resultados anormales en sus análisis de sangre. Su médico podría también realizar análisis para comprobar directamente su médula ósea durante el tratamiento con Revolade.
Revisión de hemorragias digestivas
Si está tomando interferón, tratamiento base junto con Revolade, se le realizará un seguimiento para detectar cualquier signo de hemorragia en su estómago o intestino después de que deje de tomar Revolade.
Supervisión del corazón
Su médico puede considerar, si es necesario, supervisar su corazón mientras se encuentre en tratamiento con Revolade mediante un electrocardiograma.
Personas mayores (65años y más)
Hay pocos datos sobre el uso de Revolade en pacientes de 65 años de edad o más. Si tiene 65 o más años ha de tener cuidado cuando utilice Revolade.
Niños y adolescentes
No se recomienda el uso de Revolade en niños menores de 1 año con PTI. Tampoco se recomienda en niños menores de 18 años con bajos niveles de plaquetas debidas a hepatitis C o a anemia aplásica grave.
Otros medicamentos y Revolade
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Esto incluye medicamento obtenidos sin receta y vitaminas.
Algunos medicamentos de uso común pueden interaccionar con Revolade(incluyendo medicamentos con receta médica, sin receta médica y minerales). Estos incluyen:
- medicamentos antiácidos para tratar la indigestión, ardor de estómagoo úlceras de estómago(ver también la sección 3 “Cuándo tomarlo”).
- medicamentos llamados estatinas, para disminuir el colesterol
- algunos medicamentos para tratar la infección por VIH, como lopinavir y/o ritonavir
- ciclosporina, utilizada en los trasplanteso en enfermedades inmunológicas
- minerales como el hierro, calcio, magnesio, aluminio, selenio y zinc, que pueden estar presentes en suplementos de vitaminas y minerales(ver también la sección 3 “Cuándo tomarlo”).
- medicamentos como el metotrexato y topotecan, utilizados para tratar el cáncer
Consulte con su médicosi está tomando cualquiera de estos medicamentos. Algunos no se deben tomar con Revolade, puede ser necesario ajustar la dosis o puede requerir modificar las horas en que los toma. Su médico revisará los medicamentos que está tomando y le recomendará alternativas si es necesario.
Si además está tomando medicamentos para prevenir la formación de trombos, existe un mayor riesgo de tener hemorragias. Su médico hablará de esto con usted.
Si está tomando corticosteroides, danazol,y/o azatioprinajunto con Revolade, puede que sea necesario reducir la dosis o interrupir el tratamiento de estos medicamentos.
Toma de Revolade con alimentos y bebidas
No tome Revolade con alimentos o bebidas lácteas, ya que el calcio de los productos lácteos afecta la absorción del medicamento. Para más información, ver la sección 3, “Cuándo tomarlo”.
Embarazo y lactancia
No tome Revolade si está embarazadaa menos que su médico se lo recomiende específicamente. No se conoce el efecto de Revolade durante el embarazo.
- Informe a su médico si está embarazada,cree que podría estar embarazada o tiene intención de quedarse embarazada.
- Utilice un método anticonceptivo fiablepara prevenir el embarazo mientras esté tomando Revolade.
- Si se queda embarazada durante el tratamientocon Revolade, informe a su médico.
No dé el pecho mientras está tomando Revolade. Se desconoce si Revolade pasa a la leche materna.
Si está en periodo de lactanciao planea dar el pecho, informe a su médico.
Conducción y uso de máquinas
Revolade le puede provocar mareosy tener otros efectos adversos que le hagan estar menos alerta.
No conduzca o use máquinasa menos que esté seguro de que Revolade no le afecta.
Revolade contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por comprimido; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Revolade
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico. No cambie la dosis o la pauta de tratamiento con Revolade, a menos que su médico o farmacéutico se lo aconsejen. Mientras este tomando Revolade, estará bajo la supervisión de un médico especialista con experiencia en el tratamiento de su enfermedad.
Cuánto tomar
Para PTI
Adultos y niños(de 6 a 17 años) - la dosis inicial habitual para PTI es de un comprimido de 50mgde Revolade al día. Si es una persona de ascendencia del Este o Sudeste asiático, puede necesitar iniciar el tratamiento con una dosis menor, de 25mg.
Niños(de 1 a 5 años) - la dosis inicial habitual para PTI es de un comprimido de 25mgde Revolade al día.
Para Hepatitis C
Adultos- la dosis inicial habitual para hepatitis C es de un comprimido de 25mgde Revolade al día. Si es una persona de ascendencia del Este o Sudeste asiático inicie el tratamiento con la misma dosis de25mg.
Para AAG
Adultos– la dosis inicial habitual para AAG es de un comprimido de 50 mg de Revolade al día. Si es una persona de ascendencia del Este o Sudeste asiático (puede necesitar iniciar el tratamiento con una dosis menor de 25mg.
Revolade puede tardar de 1 a 2 semana en hacerle efecto. En función de su respuesta a Revolade su médico puede recomendarle cambiar su dosis diaria.
Cómo tomar los comprimidos
Trague el comprimido entero, con agua
Cuándo tomarlo
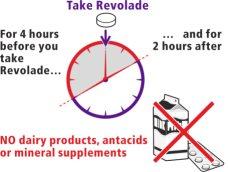
Asegurese que –
- en las 4horas antesde tomar Revolade
- y en las 2horas despuésde tomar Revolade
noconsuma nada de:
- alimentos lácteoscomo queso, mantequilla, yogur o helado
- leche o batidos de leche, bebidas hechas con leche, yogur o nata
- antiácidos, un tipo de medicamentos para la indigestión y el ardor
- algunos suplementos de vitaminas y minerales, incluyendo hierro, calcio, magnesio, aluminio, selenio y zinc.
Si lo hace, su organismo no absorberá adecuadamente el medicamento.



Para obtener más información sobre qué alimentos y bebidas son los adecuados, consulte a su médico.
Si toma más Revolade del que debe
Consulte inmediatamente a su médico o farmacéutico. Si es posible muéstrele el envase o este prospecto. Se le controlará por si aparecen signos o síntomas de efectos adversos y se le administrará inmediatamente el tratamiento adecuado.
Si olvidó tomar Revolade
Tome la siguiente dosis a la hora de siempre. No tome más de una dosis de Revolade al día.
Si interrumpe el tratamiento con Revolade
No deje de tomar Revolade sin antes consultar con su médico. Si su médico le aconseja interrumpir el tratamiento, se le controlarán los niveles de plaquetas cada semana, durante cuatro semanas. Ver también “Hemorragias o hematomas tras la interrupción del tratamiento” en la sección 4.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Síntomas a los que necesita prestar atención: acuda a su médico
Las personas que toman Revolade tanto para la PTI como para los recuentos bajos de plaquetas asociados a la hepatitis C, pueden presentar signos relacionados con posibles efectos adversos graves. Es importante que informe a su médico si desarrolla los síntomas.
Mayor riesgo de trombos
Algunas personas pueden tener mayor riesgo de tener un trombo, y los medicamentos como Revolade pueden empeorar este problema. El bloqueo repentino de un vaso sanguíneo por un trombo, es un efecto adverso poco frecuente y que puede afectar hasta 1 de cada 100 personas.
Busque ayuda médica inmediatamente si presenta sígnos o síntomas de trombo, como:
- hinchazón, dolor, calor, enrojecimiento osensibilidad en una pierna
- dificultad respiratoria repentina, excepcionalmente acompañada de dolor agudo en el pecho o respiración agitada.
- dolor abdominal (estómago),abdomen agrandado, sangre en sus heces.
Problemas de hígado
Revolade puede causar cambios que aparezcan reflejados en los análisis de sangre, y que pueden ser signos de daño hepático. Los problemas en el hígado (aumento de las enzimas hepáticoas en los análisis de sangre) son frecuentes y pueden afectar hasta 1 de cada 10 personas. Otros problemas de hígado son poco frecuentes y pueden afectar hasta 1 de cada 100 personas.
Si tiene cualquiera de los signos de problemas en el hígado:
- color amarillentoen la piel o en el área blanca de los ojos (ictericia)
- orina de un color oscuroinusual.
contacte con su médico inmediatamente
Hemorragias o hematomas tras la interrupción del tratamiento
A las dos semanas después de interrumpir el tratamiento con Revolade, normalmente sus niveles de plaquetas caerán a niveles similares a los que tenía antes de iniciar Revolade. Una disminución en los niveles de plaquetas puede aumentar el riesgo de tener hemorragias o hematomas. Su médico comprobará sus niveles de plaquetas durante al menos 4 semanas después de interrumpir el tratamiento con Revolade.
Contacte con su médicosi tiene hemorragias o hematomas al dejar de tomar Revolade.
Algunas personas tienen sangrados en elsistema digestivotras dejar de tomar peginterferón, ribavirina y Revolade. Los síntomas incluyen:
- heces negras de aspecto alquitranado (la decoloración de las heces esun efecto adverso poco frecuente que puede afectar hasta 1 de cada 100 personas).
- sangre en las heces
- vomitar sangre o algo que parecen granos de café
Contacte con su médico inmediatamentesi tiene alguno de estos síntomas.
Se han notificado los siguientes efectos adversos relacionados con el tratamiento con Revolade en pacientes adultos con PTI
Efectos adversos muy frecuentes
Pueden afectar a más de 1 de cada 10personas
- resfriado
- sensación de mareo (náuseas)
- diarrea
- tos
- infección de nariz, de los senos nasales, de la garganta y de las vías respiratorias (infección de las vías altas respiratorias)
- dolor de espalda
Efectos adversos muy frecuentes que pueden verse en los análisis de sangre
- aumento de las enzimas del hígado (alanina aminotransferasa (ALT))
Efectos adversos frecuentes
Pueden afectar hasta 1 de cada 10personas
- dolor muscular, espasmo muscular, debilidad muscular
- dolor de huesos
- menstruación abundante
- irritación de garganta y molestias al tragar
- problemas oculares incluyendo anomalía en las pruebas de ojos, ojo seco, dolor ocular y visión borrosa
- vómitos
- gripe
- herpes labial
- neumonía
- irritación e inflamación (hinchazón) de los senos
- inflamación (hinchazón) e infección en las amígdalas
- infección de los pulmones, de los senos, de la nariz y de la garganta
- inflamación de las encías
- pérdida de apetito
- sensación de hormigueo, picazón o entumecimiento
- disminución de sensibilidad en la piel
- somnoliencia
- dolor de oídos
- dolor, hinchazón y sensibilidad en una de las piernas (generalmente la pantorrilla) con la piel caliente en la zona afectada (signos de un coágulo de sangre en una vena profunda)
- hinchazón localizado relleno de sangre de una rotura de un vaso sanguíneo (hematoma)
- sofocos
- alteraciones en la boca incluyendo sequedad o irritación en la boca, sensibilidad en la lengua, sangrado en las encías, úlceras en la boca
- moqueo
- dolor de muelas
- dolor abdominal
- función hepática anormal
- cambios en la piel que incluyen sudoración excesiva, erupción con picor, manchas rojas, cambios en la apariencia de la piel
- perdida de pelo
- orina espumosa o con burbujas (signos de proteína en la orina)
- temperatura elevada, sensación de calor
- dolor en el pecho
- sensación de debilidad
- problemas para dormir, depresión
- migraña
- disminución de la visión
- sensación de que todo da vueltas (vértigo)
- gases
Efectos adversos frecuentes que pueden aparecer en los análisis de sangre:
- disminución del número de glóbulos rojos (anemia)
- disminución del número de plaquetas (trombocitopenia)
- disminución del número de glóbulos blancos
- disminución en los niveles de hemoglobina
- aumento del número de eosinófilos
- aumento del número de glóbulos blancos (leucocitosis)
- aumento del nivel de ácido úrico
- disminución de los niveles de potasio
- aumento de los niveles de creatinina
- aumento de los niveles de fosfatasa alcalina
- aumento de enzimas hepáticas (aspartato aminotransferasa (AST))
- aumento en la bilirrubina sanguínea (una sustancia producida por el hígado)
- aumento en los niveles de algunas proteínas
Efectos adversos poco frecuentes
Pueden afectar hasta1 de cada 100personas:
- reacción alérgica
- interrupción del aporte de sangre a partes del corazón
- dificultad respiratoria repentina, especialmente cuando va acompañada de dolor agudo en el pecho y/o respiración agitada, que podrían ser signos de un trombo en los pulmones (ver “Mayor riesgo de trombos” anteriormente en la sección 4)
- pérdida parcial de la función pulmonar causada por un bloqueo en la arteria pulmonar
- posible dolor, hinchazón, y/o enrojecimiento alrededor de una vena que podrían ser signos de trombos en una vena
- piel amarillenta y/o dolor abdominal que podrían ser signos de una obstrucción de un conducto biliar, lesión en el hígado, daño hepático debido a la inflamación (ver “Problemas de hígado” anteriormente en la sección 4)
- daño en el hígado debido a la medicación
- latido más rápido del corazón, latido del corazón irregular, decoloración azulada de la piel, alteraciones en el ritmo cardiaco (prolongación del intervalo QT) que podría ser signo de un desorden relacionado con el corazón y vasos sanguíneos.
- coagulos sanguíneos
- sofocos
- dolor e hinchazón de las articulaciones debido al ácido úrico (gota)
- falta de interés, cambios en el estado de ánimo, llanto difícil de calmar o que ocurre de forma inesperada
- problemas de equilibrio, alteraciones en el habla y en la función nerviosa, sacudidas
- dolor o sensaciones anormales en la piel
- parálisis de un lado del cuerpo
- migraña con aura
- dolor de nervios
- dilatación o hinchazón de los vasos sanguïneos que causan dolor de cabeza
- problemas en los ojos, incluyendo un mayor lagrimeo, enturbiamiento de la lente del ojo (cataratas), hemorragia en la retina, ojo seco
- problemas de nariz, de garganta y de senos nasales, problemas para respirar al dormir
- ampollas/dolor en boca y garganta
- pérdida de apetito
- problemas en el aparato digestivo, incluyendo movimientos intestinales frecuentes, intoxicación alimentaria, sangre en heces, vómitos de sangre
- hemorragia en el recto, cambios en el color de las heces, hinchazón abdominal, estreñimiento
- alteraciones en la boca, incluyendo sequedad o irritación en la boca, dolor en la lengua, sangrado de encías, molestias en la boca
- quemadura solar
- sentir calor, sensación de ansiedad
- enrojecimiento o inflamación alrededor de las heridas
- sangrado alrededor de un catéter (si lo tuviera) en la piel
- sensación de cuerpo extraño
- problemas de riñón incluyendo inflamación de los riñones, micción excesiva (aumento de la necesidad de orinar) durante la noche, fallo renal, glóbulos blancos en orina
- sudor frío
- sensación de malestar general
- infección en la piel
- cambios en la piel incluyendo decoloración de la piel, descamación, enrojecimiento, picor y sudoración
- debilidad muscular
- cáncer de recto y colon
Efectos adversos poco frecuentes que pueden aparecer en los análisis de sangre:
- cambios en la forma de los glóbulos blancos
- presencia de glóbulos blancos inmaduros que pueden ser indicativos de ciertas enfermedades
- aumento del número de plaquetas
- disminución de los niveles de calcio
- disminución del número de glóbulos rojos (anemia) caudas por una excesiva destrucción de glóbulos rojos (anemia hemolítica)
- aumento del número de mielocitos
- aumento de neutrófilos
- aumento de la urea en sangre
- aumento de proteínas en orina
- aumento de los niveles de albúmina en sangre
- aumento de los niveles totales de proteínas
- disminución de los niveles de albúmina en sangre
- aumento del pH en orina
- aumento de los niveles de hemoglobina
Se han notificado los siguientes efectos adversos relacionados con el tratamiento con Revolade en en niños (de 1 a 17años) con PTI
Si estos efectos adversos se agravaran, por favor informe a su médico, farmacéutico o enfermero.
Efectos adversos muy frecuentes
Pueden afectar a más de 1 de cada 10niños
- infección en la nariz, de los senos nasales, de la garganta y de las vías respiratorias altas, resfriado (infección del tracto respiratorio superior)
- diarrea
- dolor abdominal
- tos
- temperatura elevada
- sensación de mareo (náuseas)
Efectos adversos frecuentes
Pueden afectar hasta 1 de cada 10niños
- dificultad para dormir (insomnio)
- dolor de muelas
- dolor de garganta y de nariz
- picor, moqueo,o taponamiento
- irritación de garganta, moqueo, congestión nasal y estornudos
- alteraciones en la boca incluyendo sequedad, irritación en la boca, sensibilidad en la lengua, sangrado en las encías, úlceras en la boca
Se han notificado los siguientes efectos adversos relacionados con el tratamiento con Revolade en combinación con perginterferon y ribavirina en pacientes con VHC
Efectos adversos muy frecuentes
Pueden afectar a más de 1 de cada 10personas:
- dolor de cabeza
- pérdida del apetito
- tos
- sentirse mareado (nauseas), diarrea
- dolor muscular, debilidad muscular
- picor
- sensación de cansancio
- fiebre
- pérdida del pelo
- sensación de debilidad
- malestar similar al que poduce la gripe
- hinchazón de manos o pies
- escalofríos
Efectos adversos muy frecuentes que pueden aparecer en los análisis de sangre:
- disminución del número de glóbulos rojos (anemia)
Efectos adversos frecuentes
Pueden afectar hasta 1 de cada 10personas:
- infección del tracto urinario
- inflamación de los conductos nasales, garganta y boca, síntomas similares a los de la gripe, sequedad, irritación o inflamación de la boca, dolor de muelas
- pérdida de peso
- trastornos del sueño, somnolencia anormal, depresión, ansiedad
- mareos, problemas de atención y de memoria, cambios en el estado de ánimo
- disminución de la función cerebral debido a un daño en el hígado
- hormigueo o entumecimiento de manos y pies
- fiebre, dolor de cabeza
- problemas en los ojos, incluyendo enturbiamiento de la lente del ojo (cataratas), ojo seco, pequeños depósitos amarillos en la retina, color amarillento en el área blanca de los ojos
- sangrado de la retina
- sensación de que todo da vueltas
- latidos del corazón rápidos e irregulares (palpitaciones), dificultad para respirar
- tos con flemas, moqueo, gripe (influenza), herpes labial, irritación de garganta y molestias al tragar
- alteraciones del sistema digestivo, incluyendo vómitos, dolor de estómago, indigestión, estreñimiento, estómago hinchado alteraciones en el gusto, almorranas (hemorroides), dolor/malestar abdominal, hinchazón de los vasos sanguíneos y sangrado en la garganta (esófago)
- dolor de muelas
- problemas de hígado, incluyendo un tumor en el hígado, amarilleo del blanco de los ojos o piel (ictericia), daño hepático debido a medicamentos (ver “Problemas de hígado” anteriormente en la sección 4)
- cambios en la piel, incluyendo erupción, piel seca, eczema, enrojecimiento de la piel, picor, sudoración excesiva, crecimiento inusual de la piel, pérdida de cabello
- dolor de articulaciones, dolor de espalda, dolor de huesos, dolor en las extremidades (brazos, piernas, manos y pies), espasmos musculares
- irritabilidad, sensación de malestar general, reacciones cutáneas tales como enrojecimiento o hinchazón y dolor en el lugar de inyección, dolor en el pecho y molestias, retención de líquidos en el cuerpo o extremidades que causa hinchazón
- infección de nariz, de senos nasales, de garganta y de vías respiratorias, resfriado (infección de las vías altas respiratorias), inflamación de la mucosa que recubre los bronquios
- depresión, ansiedad, problemas de sueño, nerviosismo
Efectos adversos frecuentes que pueden aparecer en los análisis de sangre:
- aumento del azúcar (glucosa) en sangre
- disminución del número de glóbulos blancos
- disminución del número de neutrófilos
- disminución de la albúmina de la sangre
- disminución de los niveles de hemoglobina
- aumento de los niveles de bilirrubina sanguínea (una sustancia producida por el hígado) en sangre
- cambios en las enzimas que controlan la coagulación de la sangre
Efectos adversos poco frecuentes
Pueden afectar hasta 1 de cada 100personas:
- dolor al orinar
- alteraciones en el ritmo cardiaco (prolongación del intervalo QT)
- gripe estomacal (gastroenteritis), dolor de garganta
- ampollas/dolor en la boca, inflamación del estómago
- cambios en la piel, incluyendo cambios de color, descamación, enrojecimiento de la piel, picor, lesión y sudor nocturno
- coágulos sanguíneos en las venas del hígado (posible daño hepático y/o del sistema digestivo)
- mala coagulación en pequeños vasos sanguíneos con fallo renal
- prurito y moratones en lugar de inyección, molestias en el pecho
- disminución del número de glóbulos rojos (anemia) causada por destrucción masiva de glóbulos rojos (anemia hemolítica)
- confusión, agitación
- fallo hepático
Se han observado los siguientes efectos adversos asociados al tratamiento con Revolade en pacientes con anemia aplásica grave (AAG)
Si estos efectos adversos se agravaran, por favor informe a su médico, farmacéutico o enfermero
Efectos adversos muy frecuentes
Pueden afectar a más de 1 de cada 10personas:
- tos
- dolor de cabeza
- dolor en la boca y garganta
- diarrea
- mareo, nauseas
- dolor articular (artralgia)
- dolor en la extremidades (brazos, piernas, manos y pies)
- vértigos
- sentirse muy cansado
- fiebre
- escalofríos
- picor de ojos
- ampollas en la boca
- sangrado en las encías
- dolor abdominal
- espasmos musculares
Efectos adversos muy frecuentes que pueden aparecer en una analítica
- cambios anormales de las células de su médula ósea
- aumento de las enzimas hepáticas (aspartato aminotransferasa (AST))
Efectos adversos frecuentes
Pueden afectar hasta 1 de cada 10personas:
- ansiedad
- depresión
- sentir frío
- sensación de malestar general
- problemas en los ojos que incluyen problemas de visión, enturbiamiento de la lente del ojo (cataratas), manchas o depósitos en el ojo (cuerpos vítreos flotantes), ojo seco, picor de ojos, amarilleo del blanco de los ojos o de la piel
- sangrado de nariz
- problemas digestivos incluidos dificultad para tragar, dolor en la boca, hinchazón en la lengua, vómitos, pérdida del apetito , dolor/malestar de estómago,hinchazón de estómago, flatulencias/gases de la digestión, estreñimiento, alteraciones en la motilidad intestinal que pueden provocar estreñimiento, hinchazón, diarrea y/o los síntomas antes mencionados, cambios de coloración de las heces
- desmayos
- problemas de piel incluyendo manchas rojas o púrpuras debidas a sangrados debajo de la piel (petequias), erupción, picor, urticaria, lesiones en la piel
- dolor de espalda
- dolor muscular
- dolor de huesos
- debilidad (astenia)
- hinchazón de las extremidades inferiores debido a una acumulación de líquidos
- coloración anormal de la orina
- interrupción en la circulación al bazo (infarto esplénico)
- moqueo
Efectos adversos frecuentes que pueden aparecer en una analítica
- aumento de algunas enzimas debido a la degradación muscular (creatinina fosfoquinasa)
- acumulación de hierro en el cuerpo (sobrecarga de hierro)
- disminución de los niveles de azúcar (hipoglucemia)
- aumento de la bilirrubina en sangre (una sustancia producida por el hígado)
- disminución del número de glóbulos blancos
Efectos adversos de frecuencia no conocida
No puede estimarse la frecuencia a partir de los datos disponibles
- decoloración de la piel
- oscurecimiento de la piel
- daño hepático debido a la medicación
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Revolade
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en el blíster.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Revolade
El principio activo de Revolade es eltrombopag.
Comprimidos recubiertos con película de 12,5mg
Cada comprimido recubierto con película contiene eltrombopag olamina equivalente a 12,5 mg de eltrombopag.
Comprimidos recubiertos con película de 25mg
Cada comprimido recubierto con película contiene eltrombopag olamina equivalente a 25 mg de eltrombopag.
Comprimidos recubiertos con película de 50mg
Cada comprimido recubierto con película contiene eltrombopag olamina equivalente a 50 mg de eltrombopag.
Comprimidos recubiertos con película de 75mg
Cada comprimido recubierto con película contiene eltrombopag olamina equivalente a 75 mg de eltrombopag.
Los demás componentes son: hipromelosa, macrogol 400, estearato de magnesio, manitol (E421), celulosa microcristalina, povidona, carboximetilalmidón sódico, dióxido de titanio (E171).
Revolade 12,5 mg y 25 mg comprimidos recubiertos con película también contienen polisorbato 80 (E433).
Revolade 50 mg comprimidos recubiertos con película también contiene: óxido de hierro rojo (E172), óxido de hierro amarillo (E172).
Revolade 75 mg comprimidos recubiertos con película también contiene: óxido de hierro rojo (E172), óxido de hierro negro (E172).
Aspecto del producto y contenido del envase
Los comprimidos recubiertos con película de Revolade 12,5 mg son de color blanco, redondos, biconvexos, grabados con ‘GS MZ1’ y ’12.5’ en una cara.
Los comprimidos recubiertos con película de Revolade 25 mg son de color blanco, redondos, biconvexos, grabados con ‘GS NX3’ y ‘25’ en una cara.
Los comprimidos recubiertos con película de Revolade 50 mg son de color marrón, redondos, biconvexos, grabados con ‘GS UFU’ y ‘50’ en una cara.
Los comprimidos recubiertos con película de Revolade 75 mg son de color rosa, redondos, biconvexos, grabados con ‘GS FFS’ y ‘75’ en una cara.
Se suministran en blisters de aluminio en un estuche conteniendo 14 o 28 comprimidos recubiertos con película y envases múltiples conteniendo 84 comprimidos recubiertos con película (3 envases de 28).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Irlanda
Responsable de la fabricación
Lek d.d
Verovskova Ulica 57
Ljubljana 1526
Eslovenia
Novartis Pharmaceutical Manufacturing LLC
Verovskova Ulica 57
Ljubljana 1000
Eslovenia
Novartis Farmacéutica SA
Gran Via de les Corts Catalanes, 764
08013 Barcelona
España
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Alemania
Glaxo Wellcome S.A.
Avenida de Extremadura 3
09400 Aranda de Duero
Burgos
España
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 555 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλáδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κúπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a REVOLADE 50 MG COMPRIMIDOS RECUBIERTOS CON PELICULAForma farmacéutica: COMPRIMIDO, 12,5 mgPrincipio activo: EltrombopagFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: COMPRIMIDO, 25 mgPrincipio activo: EltrombopagFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: COMPRIMIDO, 50 mgPrincipio activo: EltrombopagFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para REVOLADE 50 MG COMPRIMIDOS RECUBIERTOS CON PELICULA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de REVOLADE 50 MG COMPRIMIDOS RECUBIERTOS CON PELICULA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes
















