
RELVAR ELLIPTA 184 MCG/22 MCG POLVO PARA INHALACION (UNIDODIS)

Cómo usar RELVAR ELLIPTA 184 MCG/22 MCG POLVO PARA INHALACION (UNIDODIS)
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Relvar Ellipta 92 microgramos/22microgramos polvo para inhalación (unidosis)
Relvar Ellipta 184 microgramos/22microgramos polvo para inhalación (unidosis)
furoato de fluticasona/vilanterol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Relvar Ellipta y para qué se utiliza
- Qué necesita saber antes de empezar a usar Relvar Ellipta
- Cómo usar Relvar Ellipta
- Posibles efectos adversos
- Conservación de Relvar Ellipta
- Contenido del envase e información adicional
Instrucciones de uso paso a paso
1. Qué es Relvar Ellipta y para qué se utiliza
Relvar Ellipta contiene dos principios activos: furoato de fluticasona y vilanterol. Existen dos concentraciones diferentes de Relvar Ellipta: furoato de fluticasona 92 microgramos/vilanterol 22 microgramos y furoato de fluticasona 184 microgramos/vilanterol 22 microgramos.
La concentración de 92/22 microgramos se utiliza para el tratamiento regular de la enfermedad pulmonar obstructiva crónica (EPOC)en adultos, así como para el tratamiento del asmaen adultos y adolescentes de 12 años de edad y mayores.
La concentración de 184/22 microgramos se utiliza para el tratamiento del asmaen adultos y adolescentes de 12 años de edad y mayores.
La concentración de 184/22 microgramos no está aprobada para el tratamiento de la EPOC.
Relvar Ellipta se debe utilizar todos los días y no únicamente cuando tiene dificultad para respirar u otros síntomas del asma y la EPOC. No se debe utilizar para aliviar un ataque repentino de ahogo o sibilancias. Si tiene este tipo de ataques debe utilizar un inhalador de “rescate” de acción rápida (como salbutamol). Contacte con su médico si no tiene un inhalador de acción rápida.
Furoato de fluticasona pertenece a un grupo de medicamentos llamados corticosteroides, a menudo llamados simplemente esteroides. Los corticosteroides reducen la inflamación. Además reducen la hinchazón (inflamación) e irritación de las pequeñas vías aéreas en los pulmones y alivian de forma gradual los problemas respiratorios. Los corticosteroides también ayudan a prevenir los ataques de asma y el empeoramiento de la EPOC.
Vilanterol pertenece a un grupo de medicamentos llamados broncodilatadores de acción prolongada. Actúa relajando los músculos de las pequeñas vías aéreas en los pulmones. Esto ayuda a abrir las vías respiratorias y facilita la entrada y salida de aire de los pulmones. Cuando se usa de forma regular, ayuda a que las pequeñas vías aéreas de los pulmones permanezcan abiertas.
El uso regular de estos dos principios activos juntos le ayudará a controlar sus dificultades respiratorias, más que cualquiera de los medicamentos por separado.
El asma,es una enfermedad pulmonar crónica grave en la que los músculos que rodean las vías respiratorias más pequeñas se estrechan (broncoconstricción) y se hinchan e irritan (inflamación). Los síntomas van y vienen e incluyen dificultad para respirar, sibilancias, opresión en el pecho y tos. Se ha demostrado que Relvar Ellipta reduce los ataques y síntomas del asma.
La enfermedad pulmonar obstructiva crónica (EPOC), es una enfermedad pulmonar crónica grave en donde las vías respiratorias se inflaman y se engrosan. Los síntomas incluyen dificultad para respirar, tos, molestias en el pecho y tos acompañada de mucosidad. Relvar Ellipta ha demostrado reducir los brotes de los síntomas que acompañan a la EPOC.
2. Qué necesita saber antes de empezar a usar Relvar Ellipta
No use Relvar Ellipta
- si es alérgicoa furoato de fluticasona, vilanterol o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si piensa que lo anterior le aplica, no use Relvar Elliptahasta haber consultado con su médico.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Relvar Ellipta:
- si tiene problemas hepáticos, ya que puede ser más propenso a tener efectos adversos. Si tiene problemas hepáticos moderados o graves, su médico limitará su dosis a la concentración más baja de Relvar Ellipta (92/22 microgramos una vez al día)
- si tiene problemas cardiacoso tensión arterial alta
- si tiene tuberculosis (TB) pulmonaro cualquier otra infección desde hace tiempo o que no haya sido tratada
- si alguna vez le han dicho que tiene diabetes o el nivel de azúcar en sangre alto
- si tiene problemasde la glándula tiroides
- si tiene el potasiode la sangre bajo
- si presenta visión borrosa u otras alteraciones visuales.
Consulte con su médicoantes de utilizar este medicamento si piensa que cualquiera de las condiciones anteriores le aplican.
Mientras esté utilizando Relvar Ellipta
Dificultades respiratorias inmediatas
Si tiene opresión en el pecho, tos, sibilancias o dificultad para respirar inmediatamente después de utilizar su inhalador Relvar Ellipta:
deje de usar este medicamentoybusque atención médica inmediatamente, ya que puede tener una afección grave llamada broncoespasmo paradójico.
- Contacte con su médico si presenta visión borrosa u otras alteraciones visuales.
- Contacte con su médico si presenta un aumento de la sed, micción frecuente o cansancio inexplicable (signos de nivel alto de azúcar en sangre).
Infección pulmonar
Si está utilizando este medicamento para el tratamiento de la EPOC, puede presentar un mayor riesgo de desarrollar una infección de los pulmones conocida como neumonía. Consulte la sección 4 para obtener información sobre los síntomas a los que debe estar atento mientras esté usando este medicamento. Consulte con su médico tan pronto como sea posible si desarrolla cualquiera de esos síntomas.
Niños y adolescentes
No administre este medicamento para el tratamiento del asma a niños menores de 12 años, o en niños y adolescentes de cualquier edad para el tratamiento de la EPOC.
Otros medicamentos y Relvar Ellipta
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Consulte con su médico o farmacéutico si no esta seguro de lo que contiene su medicamento.
Algunos medicamentos pueden afectar a la forma de actuar de este medicamento, o hacer que sea más probable que presente efectos adversos. Estos incluyen:
- medicamentos llamados betabloqueantes, como metoprolol, utilizado en el tratamiento de la tensión arterial altao enfermedades del corazón
- ketoconazol, para tratar infecciones por hongos
- ritonavir o cobicistat para tratar el VIH
- agonistas ?2-adrenérgicos de acción prolongada, como salmeterol.
Consulte con su médico o farmacéuticosi está tomando alguno de estos medicamentos.
Algunos medicamentos pueden aumentar los efectos de Relvar Ellipta, por lo que su médico le puede hacer controles minuciosos si está tomando estos medicamentos.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Si está embarazada no use este medicamento a menos que su médico se lo indique.
Se desconoce si este medicamento se excreta en la leche materna. Si está en periodo de lactancia, consulte con su médicoantes de usar Relvar Ellipta. Si está en periodo de lactancia no use este medicamento a menos que su médico se lo indique.
Conducción y uso de máquinas
Es poco problable que este medicamento afecte a su capacidad para conducir o utilizar máquinas.
Relvar Ellipta contiene lactosa
Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de utilizar este medicamento.
3. Cómo usar Relvar Ellipta
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico.En caso de duda, consulte con su médico o farmacéutico.
Asma
La dosis recomendadaen el tratamiento del asma es de una inhalación (92 microgramos de furoato de fluticasona y 22 microgramos de vilanterol) una vez al día, administrada a la misma hora cada día.
Si tiene asma grave, su médico puede decidir que se administre una inhalación del inhalador que contiene la concentración más alta (184 microgramos de furoato de fluticasona y 22 microgramos de vilanterol). Esta dosis también se utiliza una vez al día, a la misma hora cada día.
EPOC
La dosis recomendadaen el tratamiento de la EPOC es de una inhalación (92 microgramos de furoato de fluticasona y 22 microgramos de vilanterol) una vez al día, administrada a la misma hora cada día.
La concentración más alta de Relvar Ellipta (184 microgramos de furoato de fluticasona y 22 microgramos de vilanterol) no es adecuada para el tratamiento de la EPOC.
La administración de Relvar Ellipta es por inhalación oral.
Use Relvar Ellipta a la misma hora cada día, ya que es eficaz durante 24 horas
Es muy importante que utilice este medicamento todos los días, como le haya recomendado su médico. Esto le ayudará a no tener síntomas ni durante el día ni durante la noche.
Relvar Ellipta no se debe utilizar para aliviar un ataque repentino de ahogo o sibilancias. Si tiene este tipo de ataques debe utilizar un inhalador de “rescate” de acción rápida (como salbutamol).
Si siente que se queda sin respiración o tiene sibilancias más frecuentemente de lo normal, o si está utilizando su inhalador de “rescate” de acción rápida más a menudo de lo habitual, acuda a su médico.
Cómo usar Relvar Ellipta
Para obtener la información completa lea las “Instrucciones de uso paso a paso” incluidas tras la sección 6 de este prospecto.
La administración de Relvar Ellipta es solo por inhalación oral. No es necesario preparar Relvar Ellipta de ninguna forma especial, ni siquiera la primera vez que se va a utilizar.
Si los síntomas no mejoran
Si los síntomas (ahogo, sibilancias, tos) no mejoran o empeoran, o si está utilizando su inhalador de “rescate” de acción rápida más a menudo de lo habitual: contacte con su médico lo antes posible.
Si usa más Relvar Ellipta del que debe
Si accidentalmente inhala más Relvar Ellipta de lo recomendado por su médico, consulte con su médico o farmacéutico. Si es posible, muéstreles el inhalador, el envase o este prospecto. Podría notar que su corazón late más rápido de lo normal, sentirse tembloroso o tener dolor de cabeza.
Si ha utilizado más medicamento de lo indicado durante un periodo de tiempo prolongado, es especialmente importante que reciba asesoramiento de su médico o farmacéutico. Esto se debe a que dosis mayores deRelvar Ellipta pueden reducir la cantidad de hormonas esteroideas producidas de forma natural por su cuerpo.
Si olvidó usar Relvar Ellipta
No inhale una dosis doble para compensar las dosis olvidadas.Tome la siguiente dosis a su hora habitual.
Si tiene sibilancias o ahogo, o desarrolla cualquier otro síntoma de un ataque de asma, utilice su inhalador de “rescate” de acción rápida(por ejemplo salbutamol), y busque asesoramiento médico.
No deje de utilizar Relvar Ellipta sin consultar
Utilice este medicamento durante el tiempo que le haya recomendado su médico. Sólo será eficaz durante el tiempo que siga utilizándolo. No deje de utilizarlo hasta que su médico se lo indique, incluso si se encuentra mejor.
Si tiene alguna duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones alérgicas
Las reacciones alérgicas son raras (pueden afectar hasta 1 de cada 1 000 personas)
Si tras la administración de Relvar Ellipta, tiene alguno de los síntomas que figuran a continuación, deje de utilizar este medicamento y póngase en contacto con su médico inmediatamente:
- erupción en la piel (habones)o enrojecimiento
- hinchazón, algunas veces de la cara o la boca (angioedema)
- aumento de las sibilancias (sonido agudo al respirar), tos o dificultad para respirar
- sensación de debilidad repentina o mareo (que puede provocar colapso o pérdida de consciencia).
Dificultades respiratorias inmediatas
Las dificultades respiratorias inmediatas después de usar Relvar Ellipta son raras.
Si su respiración o las sibilancias empeoran inmediatamente tras el uso de este medicamento, deje de usarloy busque ayuda médicainmediatamente.
Neumonía (infección de los pulmones)en pacientes con EPOC (efecto adverso frecuente, pueden afectar hasta 1 de cada 10 personas).
Si tiene alguno de los síntomas que figuran a continuación mientras está utilizando Relvar Ellipta, consulte con su médico. Podrían ser síntomas de una infección pulmonar:
- fiebre o escalofríos
- aumento de la producción de la mucosidad, cambio en el color del moco
- aumento de la tos o aumento de la dificultad para respirar.
Otros efectos adversos:
Efectos adversos muy frecuentes
Pueden afectar a más de 1 de cada 10personas:
- dolor de cabeza
- resfriado común.
Efectos adversos frecuentes
Pueden afectar hasta 1 de cada 10personas:
- aftas, protuberancias en la boca o en la garganta causadas por una infección por hongos (candidiasis). Enjuagar la boca con agua inmediatamente después de usar Relvar Ellipta puede ayudar a que este efecto adverso no se produzca
- inflamación de los pulmones (bronquitis)
- infección de los senos nasales o garganta
- gripe
- dolor e irritación en la parte posterior de la boca y garganta
- inflamación de los senos
- picor, moqueo o nariz taponada
- tos
- alteraciones en la voz
- debilitamiento de los huesos que puede producir fracturas
- dolor de estómago
- dolor de espalda
- temperatura elevada (fiebre)
- dolor en las articulaciones
- espasmos musculares.
Efectos adversos poco frecuentes
Pueden afectar hasta 1 de cada 100personas:
- latido del corazón irregular
- visión borrosa
- aumento del nivel de azúcar en sangre (hiperglucemia).
Efectos adversos raros
Pueden afectar hasta 1 de cada 1000personas
- latido rápido del corazón (taquicardia)
- nota el latido de su corazón (palpitaciones)
- temblor
- ansiedad.
Comunicación de efectos adversos
Si experimentacualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata deposiblesefectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Relvar Ellipta
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase, la bandeja y el inhalador después de CAD. La fecha de caducidad es el último día del mes que se indica.
Mantener el inhalador dentro de la bandeja sellada para protegerlo de la humedad y solo retirar inmediatamente antes del primer uso. Una vez abierta la bandeja, el inhalador puede utilizarse durante un plazo de 6 semanas, contando desde la fecha de apertura de la bandeja. Escribir la fecha en la que se debe tirar el inhalador en el espacio designado para ello en la etiqueta. La fecha se debe anotar tan pronto como el inhalador se saque de la bandeja.
No conservar a temperatura superior a 25 ºC.
Si lo conserva en la nevera, deje que el inhalador vuelva a la temperatura ambiente durante por lo menos una horaantes de utilizarlo.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Relvar Ellipta
- Los principios activos son furoato de fluticasona y vilanterol.
- Para la dosis de 92/22 microgramos: cada inhalación proporciona una dosis liberada (dosis que sale por la boquilla) de 92 microgramos de furoato de fluticasona y 22 microgramos de vilanterol (como trifenatato).
- Para la dosis de 184/22 microgramos: cada inhalación proporciona una dosis liberada (dosis que sale por la boquilla) de 184 microgramos de furoato de fluticasona y 22 microgramos de vilanterol (como trifenatato).
- Los demás componentes son lactosa monohidrato (ver sección 2 “Relvar Ellipta contiene lactosa”) y estearato de magnesio.
Aspecto del producto y contenido del envase
Relvar Ellipta es un polvo para inhalación (unidosis).
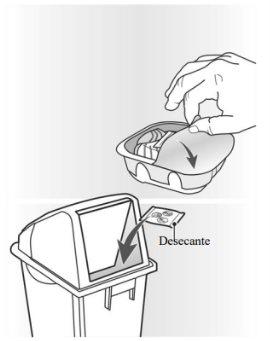
El inhalador Ellipta está formado por un inhalador de color gris claro con un protector de la boquilla de color amarillo y un contador de dosis. Está envasado en una bandeja de aluminio laminado con una tapa de aluminio desplegable. La bandeja contiene una bolsa desecante para reducir la humedad en el envase. Una vez abierta la tapa de la bandeja, tire el desecante, no lo ingiera o lo inhale. El inhalador no necesita ser conservado en la bandeja de aluminio laminado una vez que se ha abierto.
Relvar Ellipta está disponible en envases de un inhalador que contienen 14 o 30 dosis (para un tratamiento de 14 o 30 días) y en envases clínicos de 90 dosis (3 inhaladores de 30 dosis, para un tratamiento de 90 días). Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización:
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublín 24
Irlanda
D24 YK11
Responsable de la fabricación:
Glaxo Wellcome Production
Zone Industrielle No.2
23 Rue Lavoisier
27000 Evreux
Francia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals s.a./n.v. Tél/Tel: + 32 (0) 10 85 52 00 | Lietuva UAB “BERLIN-CHEMIE MENARINI BALTIC” Tel: +370 52 691 947 |
| Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals s.a./n.v. Belgique/Belgien Tél/Tel: + 32 (0) 10 85 52 00 |
Ceská republika GlaxoSmithKline s.r.o. Tel: + 420 222 001 111 | Magyarország Berlin-Chemie/A. Menarini Kft. Tel.: +36 23501301 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Trading Services Limited Tel: +356 80065004 |
Deutschland BERLIN-CHEMIE AG Tel: +49 (0) 30 67070 | Nederland GlaxoSmithKline BV Tel: + 31 (0) 33 2081100 |
Eesti OÜ Berlin-Chemie Menarini Eesti Tel: +372 667 5001 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλáδα Menarini Hellas A.E. Τηλ: +30 210 83161 11-13 | Österreich GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
España GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel.: + 48 (0)22 576 9000 |
France Laboratoire GlaxoSmithKline Tél.: + 33 (0)1 39 17 84 44 Hrvatska Berlin-Chemie Menarini Hrvatska d.o.o. Tel: +385 1 4821 361 | Portugal GlaxoSmithKline – Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 România GlaxoSmithKline Trading Services Tel: +40 800672524 |
Ireland GlaxoSmithKline Trading Services Limited Tel: + 353 (0)1 4955000 | Slovenija Berlin-Chemie / A. Menarini Distribution Ljubljana d.o.o. Tel: +386 (0)1 300 2160 |
Ísland Vistor hf. Sími: + 354 535 7000 | Slovenská republika Berlin-Chemie / A. Menarini Distribution Slovakia s.r.o. Tel: +421 2 544 30 730 |
Italia GlaxoSmithKline S.p.A. Tel: + 39 (0)45 7741111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 (0)10 30 30 30 |
Κúπρος GlaxoSmithKline Trading Services Limited Τηλ: +357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija SIA Berlin-Chemie/Menarini Baltic Tel: +371 67103210 | |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Instrucciones de uso paso a paso
¿Qué es el inhalador Ellipta?
La primera vez que utilice Relvar Ellipta, no necesita comprobar que funciona correctamente, ya viene preparado para ser utilizado directamente. Sólo siga estas instrucciones de uso paso a paso.
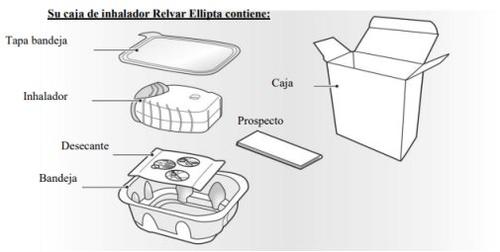
El inhalador está envasado en una bandeja. No abra la bandeja hasta que esté preparado para empezar a utilizar su nuevo medicamento. Cuando esté preparado para usar su inhalador, retire la tapa para abrir la bandeja. La bandeja contiene una bolsa desecante, para reducir la humedad. Tire la bolsa desecante, no la abra, ingiera o la inhale.
Cuando saque el inhalador de la bandeja, estará en la posición de "cerrado". No abra el inhaladorhasta que esté preparadopara inhalaruna dosis del medicamento.Cuando se abre la bandeja, se debe anotar la fecha de “Desechar el” en el espacio designado para ello que aparece en la etiqueta del inhalador. La fecha de “Desechar el” es de 6 semanas desde la fecha de apertura de la bandeja. Después de esta fecha el inhalador no debe utilizarse más. La bandeja se puede desechar después de la primera apertura.
Si se conserva en nevera, deje que el inhalador alcance la temperatura ambiente durante al menos una hora antes de su uso.
Las instrucciones de uso paso a paso que se muestran a continuación para el inhalador Ellipta de 30 dosis (30 días de tratamiento) también aplican para el inhalador Ellipta de 14 dosis (14 días de tratamiento).
- Leer las siguientes instrucciones antes de utilizarel inhalador
Si abre y cierra la tapa sin inhalar el medicamento, perderá la dosis.
La dosis perdida quedará retenida de forma segura dentro del inhalador, pero no estará disponible para ser inhalada.
No es posible administrarse accidentalmente una dosis adicional o una dosis doble mediante una inhalación.
- Preparar una dosis
Antes de abrir la tapa, espere a estar preparado para inhalar una dosis. No agite el inhalador.
- Deslice la tapa hacia abajo hasta que oiga un “clic”.
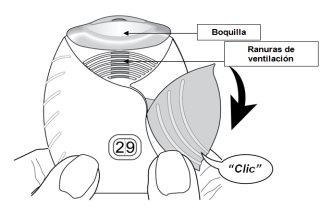
El medicamento está ahora preparado para ser inhalado.
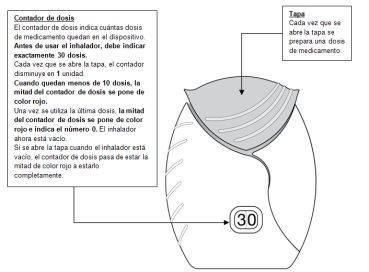
Como confirmación, el contador de dosis disminuye en 1unidad.
- Si el contador de dosis no disminuye al oír el “clic”, el inhalador no liberará el medicamento.Llévelo a su farmacéutico y solicite ayuda.
- Inhalar el medicamento
- Mantenga el inhalador alejado de la boca, espire lo que razonablemente le sea posible. Noespire dentro del inhalador.
- Coloque la boquilla entre sus labios, y ciérrelos firmemente alrededor de la boquilla.Nobloquee las ranuras de ventilación con los dedos.

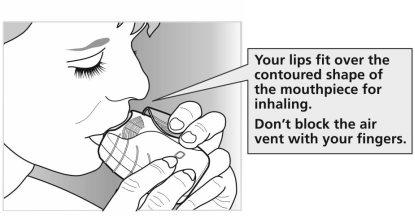
- Realice una inspiración prolongada, continua y profunda. Mantenga la respiración tanto tiempo como le sea posible (al menos 3-4 segundos).
- Retire el inhalador de la boca.
- Espire suave y lentamente.
Puede que no sea capaz de distinguir el sabor o de notar el medicamento, incluso cuando utilice el inhalador de forma correcta.
Si quiere limpiar la boquilla utilice un pañuelo seco antesde cerrar la tapa.
- Cerrar el inhalador y enjuagarse la boca
- Deslice la tapa hacia arriba hasta el tope para proteger la boquilla.
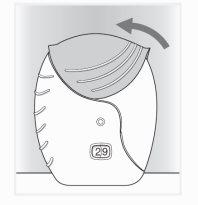
- Enjuáguese la boca con agua, una vez utilizado el inhalador, no tragar.
Esto hará que sea menos probable que se produzcan efectos adversos como ulceraciones en la boca o garganta.
- País de registro
- Precio medio en farmacia44.8 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a RELVAR ELLIPTA 184 MCG/22 MCG POLVO PARA INHALACION (UNIDODIS)Forma farmacéutica: INHALACIÓN PULMONAR, 92 microgramos/22 microgramosPrincipio activo: vilanterol and fluticasone furoateFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 184 microgramos/22 microgramosPrincipio activo: vilanterol and fluticasone furoateFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 92 microgramos/22 microgramosPrincipio activo: vilanterol and fluticasone furoateFabricante: Glaxosmithkline (Ireland) LimitedRequiere receta
Médicos online para RELVAR ELLIPTA 184 MCG/22 MCG POLVO PARA INHALACION (UNIDODIS)
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de RELVAR ELLIPTA 184 MCG/22 MCG POLVO PARA INHALACION (UNIDODIS), sujeto a valoración médica y a la normativa local.
Preguntas frecuentes
















