
QUINUX 15 MG/0,6ML SOLUCION INYECTABLE EN JERINGA PRECARGADA


Cómo usar QUINUX 15 MG/0,6ML SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
QUINUX 15 mg/0,6 ml solución inyectable en jeringa precargada
Metotrexato
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Quinux y para qué se utiliza
- Qué necesita saber antes de empezar a usar Quinux
- Cómo usar Quinux
- Posibles efectos adversos
- Conservación de Quinux
- Contenido del envase e información adicional
1. Qué es Quinux y para qué se utiliza
Quinux contiene metotrexato como principio activo.
Metotrexato es una sustancia con las siguientes propiedades:
- interfiere en el crecimiento de ciertas células del organismo que se reproducen con rapidez,
- reduce la actividad del sistema inmunitario (el mecanismo de defensa propio del organismo),
- tiene efectos antiinflamatorios.
Quinux está indicado para el tratamiento de:
- la artritis reumatoide activa en pacientes adultos,
- formas poliartríticas de artritis idiopática juvenil activa severa (AIJ), cuando la respuesta a los antiinflamatorios no esteroideos (AINE) no ha sido adecuada,
- la psoriasis grave recalcitrante e incapacitante que no responde adecuadamente a otros tratamientos tales como la fototerapia, PUVA (psoraleno + radiaciones UVA) y retinoides y la artritis psoriásica grave en pacientes adultos.
La artritis reumatoide (AR) es una enfermedad crónica del colágeno, que se caracteriza por la inflamación de las membranas sinoviales (membranas de las articulaciones). Estas membranas producen un líquido que actúa como lubricante en muchas articulaciones. La inflamación provoca el engrosamiento de la membrana y la hinchazón de la articulación.
La artritis juvenil afecta a niños y adolescentes menores de 16 años. Las formas poliartríticas están indicadas si hay afectación de 5 o más articulaciones en los primeros 6 meses de la enfermedad.
La artritis psoriásica es un tipo de artritis con lesiones psoriásicas en la piel y las uñas, especialmente en las articulaciones de los dedos de las manos y de los pies.
La psoriasis es una enfermedad crónica y frecuente de la piel que se caracteriza por unas manchas rojas cubiertas por escamas gruesas, secas, plateadas y adherentes.
Quinux modifica y enlentece la evolución de la enfermedad.
2. Qué necesita saber antes de empezar a usar Quinux
No use Quinux
- si es alérgico al metotrexato o a alguno de los demás componentes de este medicamento (incluidos en la sección 6),
- si tiene una enfermedad hepática o renal grave o enfermedades de la sangre,
- si toma habitualmente grandes cantidades de alcohol,
- si tiene una infección severa, por ejemplo, tuberculosis, VIH u otros síndromes de inmunodeficiencia,
- si tiene una úlcera en la boca, úlcera gástrica o úlcera intestinal,
- si está embarazada o en periodo de lactancia (ver sección «Embarazo, lactancia y fertilidad»),
- si recibe vacunas elaboradas con microorganismos atenuados al mismo tiempo.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Quinux:
- si tiene una edad avanzada o siente, por lo general, malestar y debilidad,
- si tiene alterada la función hepática,
- si tiene problemas de deshidratación (pérdida de líquidos).
Se ha notificado con metotrexato hemorragia pulmonar aguda en pacientes con enfermedad reumatológica subyacente. Si observa sangre al escupir o toser, se debe poner en contacto de forma inmediata con su médico.
Pruebas de seguimiento y medidas de seguridad recomendadas
Incluso cuando se administra Quinux a dosis bajas, se pueden producir efectos adversos graves. Para poder detectarlos a tiempo, es necesario que su médico le haga analíticas y chequeos.
Antes de iniciar el tratamiento con Quinux
Antes de iniciar el tratamiento, le harán análisis de sangre para comprobar que tiene suficientes células sanguíneas y pruebas para comprobar el funcionamiento del hígado y para saber si tiene hepatitis (infección hepática). Además se controlará la concentración de la albúmina sérica (una proteína de la sangre), el estado de hepatitis y el funcionamiento de los riñones. El médico también puede decidir realizar otras pruebas hepáticas, algunas de las cuales pueden ser imágenes de su hígado y otras pueden necesitar una pequeña muestra de tejido del hígado para examinarlo más de cerca. Su médico también puede comprobar si tiene tuberculosis (una enfermedad infecciosa junto con pequeños nódulos en el tejido afectado) y puede hacerle una radiografía de tórax o realizar una prueba de función pulmonar.
Durante el tratamiento
Su médico puede realizar los siguientes exámenes:
- exploración de la boca y la garganta para detectar cambios en las mucosas, como inflamación o ulceración,
- análisis de sangre/hemograma con número de células sanguíneas y medición de los niveles séricos de metotrexato,
- análisis de sangre para controlar la función hepática,
- pruebas de imagen para controlar la condición del hígado,
- pequeña muestra de tejido extraído del hígado para examinarlo más de cerca,
- análisis de sangre para controlar la función renal,
- revisión del aparato respiratorio y, si fuera necesario, la prueba de la función pulmonar.
Es muy importante que se presente a estos exámenes programados.
Si los resultados de cualquiera de estas pruebas son notorios, su médico ajustará su tratamiento en consecuencia.
El metotrexato puede afectar al sistema inmunitario y a los resultados de las vacunaciones.
También puede afectar al resultado de las pruebas inmunológicas. Puede intensificar las infecciones crónicas inactivas (por ejemplo, herpes zóster [“culebrillas”], tuberculosis, hepatitis B o C). Durante el tratamiento con Quinux no debe recibir vacunas elaboradas con microorganismos atenuados.
El metotrexato puede hacer que la piel sea más sensible a la luz solar. Evite el sol intenso y no utilice camas de bronceado ni lámparas ultravioletas sin consejo médico. Para proteger la piel del sol intenso, lleve ropa adecuada o utilice un protector solar con un factor de protección alto
Durante el tratamiento con metotrexato pueden reaparecer dermatitis producidas por la radiación y quemaduras solares (reacciones de recuerdo). Las lesiones psoriásicas pueden intensificarse durante la radiación UV (radiación ultravioleta) y la administración simultánea de metotrexato.
Puede producirse un aumento del tamaño de los nódulos linfáticos (linfoma) y, en dicho caso, el tratamiento debe suspenderse.
La diarrea puede ser un efecto tóxico de Quinux que requiere la suspensión del tratamiento. Si tiene diarrea, hable con su médico.
Se ha informado encefalopatía (un trastorno cerebral) y de leucoencefalopatía (un trastorno especial de la materia blanca del cerebro) en pacientes con cáncer que recibían tratamiento con metotrexato. Estos trastornos no pueden excluirse para el tratamiento con metotrexato en otras enfermedades.
Si usted, su pareja o su cuidador notan la aparición o un empeoramiento de síntomas neurológicos, como debilidad muscular general, alteraciones de la visión, cambios en el pensamiento, la memoria y la orientación que generan confusión y cambios en la personalidad, contacte con su médico inmediatamente porque estos pueden ser síntomas de una infección cerebral grave muy rara denominada leucoencefalopatía multifocal progresiva (LMP).
Medidas de precaución especiales para el tratamiento con Quinux
El metotrexato afecta de manera temporal a la producción de espermatozoides y óvulos, lo que es reversible en la mayoría de los casos. El metotrexato puede causar abortos y defectos de nacimiento graves. Si usted es una mujer, debe evitar quedarse embarazada mientras utilice metotrexato y durante al menos 6 meses después de haber finalizado el tratamiento. Si es un hombre, debe evitar engendrar un hijo mientras tome metotrexato y durante al menos 3 meses después de haber finalizado el tratamiento. Ver también sección «Embarazo, lactancia y fertilidad».
Pacientes de edad avanzada
Los pacientes de edad avanzada en tratamiento con metotrexato deben ser controlados de cerca por un médico para que los posibles efectos secundarios puedan detectarse lo antes posible.
El deterioro de la función hepática y renal relacionada con la edad, así como las bajas reservas corporales de ácido fólico en la vejez, requieren una dosis relativamente baja de metotrexato.
Otros medicamentos y Quinux
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
El efecto del tratamiento puede verse afectado si Quinux se administra al mismo tiempo que ciertos medicamentos:
- Medicamentos que dañan el hígado o la sangre, por ejemplo, leflunomida
- Antibióticos (medicamentos que previenen o combaten ciertas infecciones) tales como: tetraciclinas, cloranfenicol, antibióticos no absorbibles de amplio espectro, penicilinas, glucopéptidos, sulfonamidas (medicamentos que contienen sulfuro que previenen o combaten ciertas infecciones), ciprofloxacino y cefalotina
- Antiinflamatorios no esteroideos o salicilatos (medicamentos para el dolor o inflamación)
- Probenecid (medicamento para la gota)
- Ácidos orgánicos débiles como los diuréticos del asa o algunos medicamentos utilizados para el tratamiento del dolor y de enfermedades inflamatorias (por ejemplo, ácido acetilsalicílico, diclofenaco e ibuprofeno) y los pirazoles (por ejemplo, metamizol (sinónimos novaminsulfon y dipirona) para el dolor intenso y/o la fiebre)
- Medicamentos que pueden producir efectos adversos en la médula ósea, por ejemplo, el trimetoprim-sulfametoxazol (un antibiótico) y la pirimetamina
- Sulfasalazina (antirreumático)
- Azatioprina (inmunosupresor que a veces se utiliza en el tratamiento de las formas graves de la artritis reumatoide)
- Mercaptopurina (citostático)
- Retinoides (medicamentos para la psoriasis y otras enfermedades dermatológicas)
- Teofilina (medicamento para el asma bronquial y otras enfermedades pulmonares)
- Inhibidores de la bomba de protones (medicamentos para las molestias estomacales)
- Hipoglucémicos (medicamentos que se utilizan para reducir los niveles de azúcar en sangre).
Las vitaminas que contienen ácido fólico pueden alterar el efecto del tratamiento y solo se tomarán cuando lo aconseje su médico.
Deben evitarse las vacunaciones con vacunas elaboradas con microorganismos atenuados.
Uso de Quinux con alimentos, bebidas y alcohol
Durante el tratamiento con Quinux, debe evitarse el consumo de alcohol y de grandes cantidades de café, refrescos que contienen cafeína y té negro.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
No utilice Quinux durante el embarazo o si está intentando quedarse embarazada. El metotrexato puede causar defectos de nacimiento, dañar al feto o provocar abortos. Se asocia a malformaciones del cráneo, la cara, el corazón y los vasos sanguíneos, el cerebro y las extremidades. Por ello, es muy importante que no se administre metotrexato a pacientes embarazadas o que tengan previsto quedarse embarazadas. En mujeres en edad fértil se debe excluir cualquier posibilidad de embarazo con las medidas oportunas, por ejemplo, una prueba de embarazo antes de empezar el tratamiento. Debe evitar quedarse embarazada mientras toma metotrexato y durante al menos 6 meses después de suspender el tratamiento, utilizando para ello métodos anticonceptivos fiables durante todo este tiempo (ver también sección «Advertencias y precauciones»).
Si se queda embarazada durante el tratamiento o sospecha que podría estar embarazada, consulte a su médico lo antes posible. Se le debe ofrecer información sobre el riesgo de efectos perjudiciales para el niño durante el tratamiento.
Si desea quedarse embarazada, consulte a su médico, quien puede derivarle a un especialista para que la informe antes del comienzo previsto del tratamiento.
Como el metotrexato puede ser genotóxico, se recomienda a todas las mujeres que deseen quedarse embarazadas que consulten en un centro de consejo genético, si es posible, antes del tratamiento, y los hombres deben informarse sobre la posibilidad de conservar el esperma antes de comenzar el tratamiento.
Lactancia
Se debe suspender la lactancia materna durante el tratamiento con Quinux.
Fertilidad masculina
Los datos disponibles no indican un mayor riesgo de malformaciones ni abortos si el padre toma una dosis de metotrexato inferior a 30 mg/semana. Sin embargo, no se puede descartar por completo este riesgo. El metotrexato puede ser genotóxico, lo que significa que puede causar mutaciones genéticas. El metotrexato puede afectar a la producción de espermatozoides y causar defectos de nacimiento. Por tanto, debe evitar engendrar un hijo o donar semen mientras toma metotrexato y durante al menos 3 meses después de interrumpir el tratamiento.
Conducción y uso de máquinas
El tratamiento con Quinux puede producir reacciones adversas que afectan al sistema nervioso central, tales como cansancio y mareos. Por lo tanto, la capacidad para conducir o utilizar máquinas puede, en ciertos casos, verse afectada. Si se encuentra cansado o somnoliento, no debe conducir ni utilizar máquinas.
Quinux contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por unidad de dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Quinux
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Advertencia importante sobre la dosis de Quinux (metotrexato): |
Use Quinux solo una vez por semana para el tratamiento de la artritis y la psoriasis. El uso excesivo de Quinux (metotrexato) puede ser mortal. Lea la sección 3 de este prospecto con mucha atención. Si tiene alguna duda, consulte a su médico o farmacéutico antes de tomar este medicamento. |
Su médico determinará la dosis, que será ajustada de forma individual. Normalmente el tratamiento tarda entre 4 y 8 semanas en surtir efecto.
La inyección de Quinux será administrada o supervisada por su médico o profesional sanitario únicamenteuna vez a la semana. Junto con su médico, usted elegirá un día de la semana que le resulte adecuado para recibir la inyección. Quinux se puede inyectar por vía intramuscular (en un músculo) o subcutánea (bajo la piel).
Usoenniñosyadolescentes
El médico decide cuál es la dosis adecuada en niños y adolescentes con formas poliartríticas de artritis idiopática juvenil.
Quinux no está recomendado para uso en niños menores de 3 años de edad debido a que la experiencia es limitada en este grupo de edad.
Duración y forma de administración
Quinux se inyecta una vez a la semana.
El médico al cargo decidirá la duración del tratamiento. El tratamiento de la artritis reumatoide, artritis idiopática juvenil, psoriasis vulgaris y artritis psoriásica con Quinux es un tratamiento a largo plazo.
Al comienzo del tratamiento, Quinux podrá ser inyectado por el personal médico. Sin embargo, es posible que su médico decida que usted puede aprender a inyectarse Quinux usted mismo. Recibirá la formación adecuada para ello. En ninguna circunstancia debe intentar inyectarse usted mismo, a menos que se le haya enseñado a hacerlo.
Consulte las instrucciones de uso al final del prospecto.
La forma de manipular y eliminar el producto se hará conforme a las directrices de otros preparados citotóxicos de acuerdo con la normativa local. El personal sanitario gestante no debe manipular ni administrar Quinux.
El metotrexato no debe entrar en contacto con la superficie de la piel o las mucosas. Si entra en contacto, se debe aclarar inmediatamente el área afectada con abundante agua.
Si tiene la impresión de que el efecto de Quinux es demasiado fuerte o demasiado débil, consulte a su médico o farmacéutico.
SiusamásQuinuxdelquedebe
En caso de sobredosis o ingesta accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20.
SiolvidóusarQuinux
No use una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda del uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
La frecuencia y el grado de severidad de los efectos adversos dependerán de la dosis y de la frecuencia de administración. Es importante que el médico le realice controles periódicos, puesto que pueden ocurrir efectos adversos graves incluso con las dosis más bajas. Su médico le realizará pruebas para controlar anormalidades que se produzcan en la sangre como niveles bajos de leucocitos (glóbulos blancos), plaquetas, linfoma y cambios en los riñones y en el hígado.
Los efectos adversos más relevantes relacionados con la administración de Quinux ocurren en el sistema de producción de sangre y en el tubo digestivo.
Comunique a su médico inmediatamente si experimenta cualquiera de los siguientes síntomas, ya que puede indicar un efecto adverso grave con potencial peligro vital que puede requerir un tratamiento específico urgente:
- Molestias pulmonares graves (los síntomas pueden ser malestar general, tos irritante y seca, dificultad para respirar, sensación de falta de aire en reposo, dolor en el pecho o fiebre).
- Sangre al escupir o toser.
- Formación de ampollas o descamación de la piel grave.
- Hemorragias no habituales (incluidos vómitos con sangre) o hematomas.
- Diarrea grave.
- Úlceras en la boca.
- Heces negras.
- Sangre en la orina o en las heces.
- Pequeñas manchas rojas en la piel.
- Fiebre.
- Coloración amarilla de la piel (ictericia).
- Dolor o dificultad para orinar.
- Sed y/o necesidad de orinar con frecuencia.
- Ataques (convulsiones).
- Pérdida de consciencia.
- Visión borrosa o disminución de la visión.
Pueden ocurrir los siguientes efectos adversos:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- Inflamación de la boca, indigestión, náuseas (ganas de vomitar), pérdida del apetito.
- Aumento de las enzimas hepáticas.
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- Úlceras bucales, diarrea.
- Erupción, enrojecimiento de la piel, picor.
- Dolor de cabeza, cansancio, somnolencia.
- Disminución de la formación de glóbulos rojos con disminución en el número de glóbulos blancos, rojos o plaquetas (leucopenia, anemia, trombocitopenia).
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- Inflamación de la garganta, inflamación del intestino, vómitos.
- Reacciones similares a quemaduras solares debido a una mayor sensibilidad de la piel a la luz solar, caída del pelo, aumento del número de nódulos reumáticos, herpes zóster, inflamación de los vasos sanguíneos, erupción tipo herpes, urticaria.
- Aparición de diabetes mellitus.
- Mareos, confusión, depresión.
- Inflamación y úlcera de la vejiga urinaria o vagina, disminución de la función renal, trastornos urinarios.
- Dolor en las articulaciones, dolor muscular, osteoporosis (reducción de la masa ósea).
- Disminución de la albúmina sérica.
Raros(pueden afectar hasta 1 de cada 1.000 personas):
- Aumento de la pigmentación de la piel, acné, moratones debidos a la hemorragia de los vasos.
- Reacciones alérgicas, shock alérgico, inflamación alérgica de los vasos sanguíneos, fiebre, ojos rojos, infección, toxicidad en la sangre, alteraciones en la curación de las heridas, disminución del número de anticuerpos en la sangre.
- Trastornos visuales.
- Inflamación del saco alrededor del corazón, acumulación de líquido en el saco alrededor del corazón.
- Tensión arterial baja.
- Fibrosis pulmonar, neumonía causada por un microorganismo específico (neumonía por Pneumocystis carinii), dificultad respiratoria y asma bronquial, acumulación de líquido del saco alrededor del pulmón.
- Alteraciones de los electrolitos.
Muy raros(pueden afectar hasta 1 de cada 10.000 personas):
- Hemorragia profusa, megacolon tóxico (dilatación tóxica y aguda del intestino).
- Síndrome de piel escaldada, aumento de la pigmentación de las uñas, inflamación de las cutículas, furunculosis (infección profunda de los folículos del pelo), agrandamiento visible de los vasos sanguíneos pequeños.
- Lesión local en el lugar de administración (formación de abscesos estériles, cambios en el tejido graso) tras la inyección en un músculo o debajo de la piel.
- Alteración de la visión, dolor, pérdida de fuerza o sensación de entumecimiento u hormigueo/sensibilidad a los estímulos menor de la normal, alteraciones del gusto (sabor metálico), convulsiones, parálisis, dolor de cabeza fuerte con fiebre.
- Retinopatía (trastorno no inflamatorio de los ojos).
- Caída brusca en el número de glóbulos blancos, depresión severa de la médula ósea.
- Trastornos linfoproliferativos (aumento excesivo de glóbulos blancos).
- Pérdida del apetito sexual, impotencia, aumento de las mamas masculinas (ginecomastia), formación alterada del esperma, trastornos menstruales, secreción vaginal.
- Aumento del tamaño de los nódulos linfáticos (linfoma).
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- Leucoencefalopatía (una enfermedad de la materia blanca del cerebro).
- Hemorragia pulmonar.
- Lesión en los huesos de la mandíbula (secundaria a un aumento excesivo de glóbulos blancos).
- Enrojecimiento y descamación de la piel.
- Destrucción del tejido en el lugar de la inyección.
- Hinchazón.
Cuando se administra metotrexato por vía intramuscular, se pueden producir con frecuencia reacciones adversas locales (sensación de quemazón) o lesiones (formación de abscesos estériles, destrucción del tejido graso) en el lugar de la administración. La administración subcutánea de metotrexato se tolera localmente bien. Únicamente se observaron reacciones cutáneas locales leves, que disminuyeron durante el tratamiento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano. https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Quinux
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar por encima de 25 ºC. Conservar las jeringas precargadas en el embalaje exterior para protegerlas de la luz. No refrigerar o congelar.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGREde la farmacia o cualquier otro sistema de recogida de residuos de medicamentos. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Quinux
- El principio activo es metotrexato. 1 ml de solución contiene 25 mg de metotrexato (equivalente a 27,42 mg de metotrexato disódico).
1 jeringa precargada de 0,6 ml de solución contiene 15 mg de metotrexato.
- Los demás componentes son cloruro de sodio, hidróxido de sodio, agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Las jeringas precargadas de Quinux contienen una solución amarillenta y transparente.
Cada envase contiene 1 o 4 jeringa(s) precargadas(s) con 0,6 ml de solución, con agujas de inyección subcutánea acopladas y algodones impregnados en alcohol.
Cada envase contiene 1 o 4 jeringa(s) precargadas(s) con 0,6 ml de solución, con agujas de inyección subcutánea acopladas con sistema de seguridad y algodones impregnados en alcohol.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Especialidades Farmacéuticas Centrum, S.A.
C/ Sagitario, 14
03006, Alicante
EspañaGrupo Asacpharma
Tel.: 965288160; Fax.: 965286434
Responsable de la fabricación
Especialidades Farmacéuticas Centrum, S.A.
C/ Sagitario, 14
03006, Alicante
España
Fecha de la última revisión de este prospecto: octubre 2024.
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es)
Instrucciones de uso
Lea cuidadosamente las instrucciones antes de comenzar a administrar la inyección, y utilice siempre la técnica de aplicación aconsejada por su médico, enfermero o farmacéutico.
Si tiene algún problema o consulta, comuníquese con su médico, enfermero o farmacéutico.
Preparación
Seleccione una superficie de trabajo limpia, plana y bien iluminada.
Reúna todos los elementos necesarios antes de empezar:
- 1 jeringa precargada de Quinux
- 1 algodón impregnado en alcohol (que se suministra en el envase)
Lávese cuidadosamente las manos. Antes de usarla, revise la jeringa de Quinux para detectar defectos (o grietas) visibles.
Lugar de inyección
Los mejores lugares para la inyección son:
- la parte superior del muslo,
- el abdomen, salvo el área alrededor del ombligo.
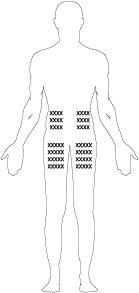
- Si alguien le está ayudando con la inyección, también puede aplicarle la inyección en el dorso del brazo, justo por debajo del hombro.
- Cambie el lugar de aplicación en cada inyección. Esto puede reducir el riesgo de desarrollar irritaciones en el lugar de inyección.
- Nunca aplique la inyección en piel dolorosa, amoratada, enrojecida, endurecida o con cicatrices o estrías. Si tiene psoriasis, no debe intentar inyectar directamente en lesiones o parches de piel elevados, engrosados, enrojecidos o escamosos.
Inyección de la solución
- Abra la caja que contiene la jeringa precargada de metotrexato y lea cuidadosamente el prospecto. Retire la jeringa precargada del envase a temperatura ambiente.
- Desinfección: seleccione un lugar para la inyección y desinféctelo con el algodón impregnado en alcohol. Deje pasar 60 segundos para que el desinfectante se seque.

- Retire cuidadosamente el capuchón de plástico protector, gírelo suavemente con un movimiento hacia fuera. Importante: no toque la aguja de la jeringa precargada.
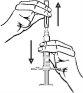
- Inserción de la cánula: con dos dedos, forme un pliegue en la piel e inserte rápidamente la aguja en un ángulo de 90 grados.
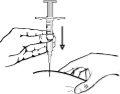
- Inyección: inserte totalmente la aguja en el pliegue de piel. Empuje lentamente el émbolo e inyecte el líquido bajo la piel. Sostenga firmemente la piel hasta que se haya completado la inyección. Retire la aguja con cuidado en línea recta.
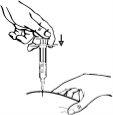
El metotrexato no debe entrar en contacto con la superficie de la piel o las mucosas. En caso de contaminación, deberá enjuagarse de inmediato el área infectada con abundante agua.
Si usted u otra persona de su entorno se lastiman con la aguja, consulte de inmediato a su médico y no utilice esta jeringa precargada.
Eliminación y otras manipulaciones
La manipulación y la eliminación del medicamento y la jeringa precargada se realizarán de acuerdo con la normativa local para agentes citotóxicos. El personal sanitario gestante no deberá manipular ni administrar Quinux.
- País de registro
- Precio medio en farmacia20.23 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a QUINUX 15 MG/0,6ML SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 10 mg/ 1 mlPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere recetaForma farmacéutica: INYECTABLE, 15 mgPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere recetaForma farmacéutica: INYECTABLE, 20 mgPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere receta
Médicos online para QUINUX 15 MG/0,6ML SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de QUINUX 15 MG/0,6ML SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






