PROLUTEX 25 MG SOLUCION INYECTABLE

Cómo usar PROLUTEX 25 MG SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para la usuaria
Prolutex 25 mg solución inyectable
Progesterona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Prolutex y para qué se utiliza
- Qué necesita saber antes de empezar a usar Prolutex
- Cómo usar Prolutex
- Posibles efectos adversos
- Conservación de Prolutex
Contenido del envase e información adicional
1. Qué es Prolutex y para qué se utiliza
Prolutex contiene el principio activo progesterona. La progesterona es una hormona sexual femenina natural. El medicamento actúa sobre el revestimiento de la matriz y le ayuda a quedarse y permanecer embarazada.
Prolutex está indicado para mujeres que necesitan progesterona extra mientras están bajo tratamiento en un protocolo de Técnicas de Reproducción Asistida (TRA), y que no pueden utilizar o tolerar preparaciones vaginales.
2. Qué necesita saber antes de empezar a usar Prolutex
No use Prolutex
- si es alérgica a la progesterona o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6);
- si tiene sangrado vaginal inusual que no ha sido valorado por su médico;
- si tiene un aborto y su médico sospecha que algún tejido está todavía en la matriz;
- si tiene actualmente un embarazo fuera de la matriz (embarazo ectópico);
- si tiene actualmente o ha tenido problemas graves de hígado;
- si tiene o sospecha cáncer de mama o del tracto genital;
- si tiene o ha tenido coágulos sanguíneos en las piernas, pulmones, ojos o en cualquier parte del cuerpo;
- si tiene porfiria (un grupo de trastornos heredados o adquiridos de ciertas enzimas);
- si durante el embarazo usted ha tenido ictericia (coloración amarillenta de los ojos y la piel debido a problemas de hígado), picazón intensa y/o ampollas en la piel;
- si tiene menos de 18 años de edad.
Advertencias y precauciones
Tenga especial cuidado con Prolutex
Si experimenta cualquiera de los siguientes trastornos durante el tratamiento, informe a su médico inmediatamente, ya que puede necesitar interrumpirse. Informe también a su médico de inmediato si los experimenta a los pocos días después de la última dosis:
- ataque al corazón (dolores en el pecho o espalda, y/o dolor profundo y dolor pulsátil en uno o en ambos brazos, dificultad repentina para respirar, sudoración, mareos, vahído, náuseas, palpitaciones);
- accidente cerebrovascular (cefalea o vómitos intensos, mareos, desvanecimiento o cambios en la visión o el habla, debilidad o entumecimiento en un brazo o una pierna);
- coágulos de sangre en los ojos o en cualquier parte del cuerpo (dolor en los ojos o dolor e hinchazón en los tobillos, los pies y las manos);
- empeoramiento de los síntomas de depresión.
- dolor de cabeza intenso, cambios en la visión
Antes de comenzar el tratamiento
Antes del tratamiento con Prolutex consulte con su médico si tiene o ha tenido cualquiera de los siguientes problemas de salud:
- Problemas hepáticos (leves o moderados)
- Epilepsia
- Migraña
- Asma
- Trastornos cardíacos o renales
- Diabetes
- Depresión
Si este fuera su caso, su médico le hará un especial seguimiento durante el tratamiento.
Niños y adolescentes
El medicamento no debe usarse en niños ni adolescentes
Uso de Prolutex con otros medicamentos
Comunique a su médico o farmacéutico que está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento, incluyendo medicamentos sin prescripción médica o medicamentos a base de plantas. Algunos medicamentos podrían interaccionar con Prolutex. Por ejemplo:
- Carbamazepina (usada para tratar crisis/ataques)
- Rifampicina (antibiótico)
- Griseofulvina (medicamento antimicótico)
- Fenitoína y fenobarbital (usados para tratar la epilepsia)
- Medicamentos a base de plantas que contienen hierba de San Juan
- Ciclosporina (medicamento para algunos tipos de inflamaciones y para después de los trasplantes de órganos)
- Medicamentos para la diabetes
- Ketoconazol (medicamento antimicótico)
No administrar Prolutex al mismo tiempo que otro medicamento inyectable.
Embarazo y lactancia
Si está embarazada o en período de lactancia, o cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Prolutex puede utilizarse durante el primer trimestre del embarazo.
Este medicamento no debe ser utilizado durante la lactancia.
Conducción y uso de máquinas
No conduzca ni utilice herramientas o máquinas si siente somnolencia y/o mareos mientras usa Prolutex
Prolutex contiene Hidroxipropil betadex
Si padece una insuficiencia renal, consulte con su médico antes de tomar este medicamento.
Prolutex contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por unidad de dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Prolutex
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
¿Qué cantidad de Prolutex debe usar y durante cuánto tiempo?
La dosis habitual es de una inyección diaria de 25 mg normalmente hasta las 12 semanas después del embarazo confirmado (por ejemplo, 10 semanas de tratamiento).
Como debe administrarse Prolutex
Prolutex puede administrarse por debajo de la piel (inyección subcutánea) o en el músculo (inyección intramuscular).
Podrá autoadministrarse 25 mg de Prolutex, por vía subcutánea, después de recibir el asesoramiento y formación adecuadas por parte del médico o el profesional sanitario.
Inyección subcutánea
Antes de autoadministrarse una inyección de Prolutex recibirá la siguiente formación y asesoramiento:
- práctica de la administración de inyecciones subcutáneas;
- dónde inyectar el medicamento;
- cómo preparar la solución inyectable;
- cómo administrar el medicamento.
Lea las instrucciones a continuación sobre la preparación y la administración de Prolutex.
Los pasos a seguir para la correcta autoadministración son:
- Preparación de la inyección
- Comprobación del material
- Preparación del vial y la jeringa
- Llenado de la jeringa
- Cambio de la aguja de inyección
- Eliminación de las burbujas de aire
- Inyección mediante administración por vía subcutánea
- Eliminación de los materiales usados
Estos pasos se explican con detalle a continuación.
IMPORTANTE: cada vial sólo debe utilizarse una vez. La solución debe utilizarse inmediatamente después de abrir el vial. No debe almacenarse en la jeringa.
- Preparación de la inyección
Es importante mantenerlo todo lo más limpio posible, así que, comience lavándose las manos completamente y séquelas con una toalla limpia. Seleccione un área limpia para preparar el medicamento:
- Un vial conteniendo Prolutex solución inyectable
Los siguientes materiales NOse suministran con su medicamento. Su médico o farmacéutico suministrará estos materiales.
- Una jeringa
- Una aguja grande (típicamente aguja verde 21G; para la administración intramuscular)
- Una pequeña aguja fina (típicamente una aguja gris 27G; para inyección subcutánea)
- Dos toallitas con alcohol
- Un recipiente para objetos cortantes (para la eliminación segura de agujas, viales, etc.)
- Comprobación del material
- El vial de Prolutex, la jeringa y las agujas llevan cubiertas protectoras.
- Compruebe que todas las cubiertas estén en buenas condiciones y no las utilice si están dañadas.
- Asegúrese que la fecha de caducidad aún sea válida en el vial de Prolutex. No utilice los productos fuera de la fecha de caducidad.
- Preparación del vial y la jeringa
|
|
- Llenado de la jeringa
|
|
- Cambio de la aguja de inyección
Este paso sólo se requiere si realiza una administración por vía subcutánea; si su médico realiza una inyección intramuscular, pasará a fijar la dosis para administrar la inyección.
- Ponga la cubierta de la aguja en la aguja verde 21G grande y entonces quite suavemente la aguja grande de la jeringa.
- Quite la aguja de inyección gris 27G más pequeña de su embalaje, manteniendo puesta la cubierta de la aguja.
- Ponga la aguja gris 27G pequeña en la jeringa y entonces quite la cubierta de la aguja.
- Eliminación de las burbujas de aire
|
|
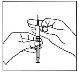
- Inyección mediante administración por vía subcutánea
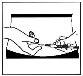
- Su médico o profesional sanitario ya le habrá mostrado dónde inyectar Prolutex (p. ej., en el abdomen o en la parte frontal del muslo).
- Abra la toallita con alcohol y limpie con cuidado el área de piel a inyectar, y deje que se seque.
- Sostenga la jeringa en una mano. Utilice la otra mano para pellizcar suavemente la piel en el área del lugar de inyección entre el pulgar y el índice.
| Mediante un movimiento de dardo, inserte la aguja gris 27G pequeña de grado fino en la piel, de modo que la piel y la aguja formen un ángulo recto. |
- Inserte la aguja gris 27G pequeña, completamente en la piel. No inyecte directamente en una vena.
- Inyecte la solución empujando suavemente el émbolo en un movimiento lento y sostenido hasta que se inyecte toda la solución debajo de la piel. Inyecte toda la solución prescrita.
- Suelte la piel y retire la aguja directamente.
- Limpie la piel en el lugar de inyección con un algodón con alcohol mediante un movimiento circular.
- Eliminación de los materiales usados
- Una vez que haya terminado con la inyección, ponga todas las agujas, viales vacíos y jeringas en un recipiente para objetos cortantes.
- Cualquier solución no utilizada debe desecharse.
La administración intramuscular debe realizarse únicamente por un médico o profesional sanitario
Todas las inyecciones intramusculares serán realizadas por un médico u otro profesional sanitario.
La inyección de Prolutex se realizará en un costado del muslo o el glúteo. Su médico o profesional sanitario limpiará el área de la piel a inyectar utilizando una toallita con alcohol, y la dejará secar. Mediante un movimiento de dardo, insertará la aguja grande dentro del músculo. Inyectará la solución empujando suavemente el émbolo en un movimiento lento y sostenido hasta que toda la solución se introduzca en el músculo. Sacará la aguja directamente y limpiará la piel en el lugar de inyección con una toallita con alcohol.
Si usa más Prolutex del que debe
Si usted ha utilizado más Prolutex del que debe, consulte inmediatamente a su médico o farmacéutico. Los síntomas de una sobredosis incluyen adormecimiento.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 915 620 420, indicando el medicamento y la cantidad utilizada.
Si olvidó usar Prolutex
No use una dosis doble para compensar la dosis olvidada.
Use la dosis tan pronto como lo recuerde y entonces prosiga como antes. Informe a su médico de lo que ha hecho.
Si interrumpe el tratamiento con Prolutex
No deje de utilizar Prolutex sin consultarlo primero con su médico o farmacéutico. La interrupción súbita de Prolutex puede causar un aumento en la ansiedad, el mal humor, y aumentar el riesgo de tener crisis (ataques).
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Prolutex puede producir efectos adversos, aunque no todas las personas los sufran.
Deje de tomar este medicamento y busque ayuda médica inmediatamente si tiene alguno de los siguientes síntomas:
- Hiperestimulación de los ovarios (los síntomas incluyen dolor en parte baja del estómago, sensación de sed y mareo y, algunas veces, vómitos, evacuación de pequeñas cantidades de orina concentrada y aumento de peso),
- Depresión,
- Coloración amarillenta de la piel y la parte blanca de los ojos (ictericia),
- Reacción alérgica grave que puede causar dificultades para respirar, hinchazón de la cara y garganta o una erupción grave (reacciones anafilactoides).
Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- dolor, enrojecimiento, picazón, irritación o hinchazón en el lugar de la inyección,
- espasmo uterino,
- sangrado vaginal.
Frecuentes:pueden afectar hasta 1 de cada 10 personas
- cefalea,
- estómago abultado,
- dolor de estómago,
- estreñimiento,
- vómito y sensación de mareo,
- dolor mamario a la palpación y/o dolor de mama,
- secreción vaginal,
- un cosquilleo o irritación molesta o picazón de la piel de la vagina y del área circundante,
- endurecimiento del área alrededor del lugar de la inyección,
- cardenales alrededor del lugar de inyección,
- fatiga (cansancio excesivo, agotamiento, letargia).
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- cambios de humor,
- mareo,
- insomnio,
- trastornos estomacales e intestinales (incluidas molestias en el estómago y/o dolor a la palpación, gases, espasmos dolorosos y arcadas),
- erupciones cutáneas (incluidas piel caliente enrojecida, abultamientos elevados, con comezón o ronchas, o piel seca, agrietada o con ampollas o hinchada),
- hinchazón y/o aumento de tamaño de la mama,
- sensación de calor,
- sensación general de molestia o "sensación de indisposición",
- dolor.
No conocida:la frecuencia no puede estimarse a partir de los datos disponibles
Los siguientes trastornos, aunque no se comunicaron por parte de los pacientes en los estudios clínicos que utilizaron Prolutex, se han descrito con otras progestinas: incapacidad para dormir (insomnio), síndrome pseudopremenstrual o trastornos menstruales, urticaria, acné, crecimiento excesivo del cabello, pérdida del cabello (alopecia) y ganancia de peso.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Conservación de Prolutex
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar por debajo de 25 °C. No refrigerar o congelar.
Conservar en el embalaje original para protegerlo de la luz.
El medicamento debe utilizarse inmediatamente después de la primera apertura.
Cualquier solución sobrante debe desecharse.
No utilice este medicamento después la fecha de caducidad que aparece en el envase después de CAD. Si la fecha de caducidad se indica como mes/año, la fecha de caducidad es el último día del mes que se indica.
No utilice este medicamento si observa partículas en la solución o si la solución no es transparente.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Prolutex
El principio activo es progesterona. Cada vial (1,112 ml) contiene 25 mg de progesterona (concentración teórica: 22,48 mg/ml)
Los demás componentes son hidroxipropilbetadex, fosfato disódico, dihidrogenofosfato de sodio dihidrato y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Prolutex es una solución incolora y transparente suministrada en un vial de vidrio incoloro.
Cada envase contiene 1, 7 ó 14 viales.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización:
IBSA Farmaceutici Italia Srl
Via Martiri di Cefalonia 2
26900 Lodi
Italia
Responsable de la Fabricación:
IBSA Farmaceutici Italia Srl
Via Martiri di Cefalonia 2
26900 Lodi
Italia
IBSA Pharma Limited (Sólo en Reino Unido)
Units 4-6 Colonial Business Park, Colonial Way
Watford WD24 4PR
Reino Unido
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Instituto Bioquimico Iberico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (España)
Este medicamento está autorizado en los Estados miembros del EEE con los siguientes nombres: (La concentración y la forma farmacéutica son idénticas en todos los países, sólo cambia el nombre comercial)
Austria: Progedex
Bélgica: Inprosub
Bulgaria: Prolutex
Chipre: Prolutex
República Checa: Prolutex
Dinamarca: Prolutex
Estonia: Lubion
Finlandia: Prolutex
Francia: Progiron
Alemania: Prolutex
Grecia: Prolutex
Hungría: Prolutex
Italia: Pleyris
Lituania: Lubion
Letonia: Lubion
Luxemburgo: Inprosub
Noruega: Prolutex
Polonia: Prolutex
Portugal: Prolutex
Rumanía: Prolutex
Eslovaquia: Prolutex
Suecia: Prolutex
Países Bajos: Prolutex
Reino Unido: Lubion
Fecha de la última revisión de este prospecto: Septiembre 2024
Si este prospecto es difícil de ver o leer, o lo querría en un formato diferente, póngase en contacto con IBSA Farmaceutici Italia Srl, Via Martiri di Cefalonia 2, 26900 Lodi, Italia (teléfono +39(0) 371 417354, e-mail [email protected] ).La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a PROLUTEX 25 MG SOLUCION INYECTABLEForma farmacéutica: OVULO/CAPSULA/COMPRIMIDO VAGINAL, 400 mgPrincipio activo: ProgesteronaFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: GEL, 1000 mg progesterona/ 100 gPrincipio activo: ProgesteronaFabricante: Seid S.A.Requiere recetaForma farmacéutica: CAPSULA, 100 mgPrincipio activo: ProgesteronaFabricante: Exeltis Healthcare S.L.Requiere receta
Médicos online para PROLUTEX 25 MG SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de PROLUTEX 25 MG SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes