
PROLASTINA 5.000 MG POLVO Y DISOLVENTE PARA SOLUCIÓN PARA PERFUSIÓN


Cómo usar PROLASTINA 5.000 MG POLVO Y DISOLVENTE PARA SOLUCIÓN PARA PERFUSIÓN
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Prolastina 4000 mg polvo y disolvente para solución para perfusión
Prolastina 5000 mg polvo y disolvente para solución para perfusión
Principio activo: alfa1-antitripsina humana
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Prolastina y para qué se utiliza
- Qué necesita saber antes de empezar a usar Prolastina
- Cómo usar Prolastina
- Posibles efectos adversos
- Conservación de Prolastina
- Contenido del envase e información adicional
1. Qué es Prolastina y para qué se utiliza
Prolastina pertenece a un grupo de medicamentos denominados “Inhibidores de proteasas”.
La alfa1-antitripsina es un componente normal de la sangre humana, cuya función consiste en inhibir la actividad de algunos enzimas como las elastasas que puede dañar los pulmones. Cuando hay una deficiencia hereditaria de alfa1-antitripsina, hay un desequilibrio entre la alfa1-antitripsina y las elastasas. Esto puede llevar a una destrucción progresiva del tejido pulmonar y desarrollar un enfisema pulmonar. El enfisema pulmonar es un agrandamiento anormal del pulmón, acompañado por destrucción del tejido pulmonar. Prolastina está indicada para restaurar el equilibrio entre alfa1‑antitripsina y las elastasas en el pulmón, y en consecuencia, evitar un mayor deterioro en el enfisema pulmonar.
Prolastina se utiliza como tratamiento crónico en cierto tipo de pacientes con déficit de alfa1‑antitripsina según es determinado por su médico.
2. Qué necesita saber antes de empezar a usar Prolastina
No use Prolastina
- Si es alérgico (hipersensible) a la alfa1-antitripsina o a cualquiera de los demás componentes de Prolastina (incluidos en la sección 6).
- Si tiene déficit de unas inmunoglobulinas determinadas, conocidas como IgA, ya que pueden desarrollarse reacciones alérgicas graves que pueden desembocar, incluso, en shock anafiláctico.
Advertencias y precauciones
- Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Prolastina.
- Comunique a su médico si padece insuficiencia cardíaca grave (fallo cardíaco). Se precisará extremar la precaución, ya que Prolastina puede producir un aumento transitorio del volumen de sangre.
Reacciones alérgicas (hipersensibilidad)
Raramente pueden presentarse reacciones de hipersensibilidad a Prolastina aunque se le hayan administrado previamente inhibidores de alfa1-antitripsina humanos y los haya tolerado bien.
Su médico le informará acerca de los síntomas de las reacciones alérgicas y le dirá qué debe hacer en caso de que los experimente (ver también la sección 4).
Si experimenta cualquier síntoma de una reacción alérgica de hipersensibilidad durante la perfusión del medicamento, advierta a su médico o enfermero de inmediato.
Información sobre seguridad con respecto al riesgo de infecciones
Para prevenir la transmisión de enfermedades infecciosas debidas al uso de medicamentos derivados de sangre o plasma humanos, se toman medidas estándar que incluyen:
- la selección cuidadosa de los donantes de sangre y plasma para garantizar la exclusión de donantes con riesgo de padecer infecciones,
- el análisis de cada donación y mezcla de plasmas para detectar posibles virus o infecciones
- la inclusión de etapas en el proceso de fabricación para inactivar/eliminar virus.
A pesar de esto, cuando se administran medicamentos derivados de sangre o plasma humano la posibilidad de transmisión de agentes infecciosos no se puede excluir totalmente. Esto también se refiere a virus y otros patógenos emergentes o de naturaleza desconocida.
Estos procedimientos se consideran efectivos frente a virus envueltos, como el virus de la inmunodeficiencia humana (VIH), el virus de la hepatitis B y el de la hepatitis C. Los procedimientos de inactivación/eliminación pueden tener un valor limitado para virus no envueltos tales como el virus de la hepatitis A o el parvovirus B19. La infección por el parvovirus B19 puede tener consecuencias graves en mujeres embarazadas (infección fetal), y en pacientes con inmunodeficiencia o con eritropoyesis aumentada (por ejemplo, en caso de anemia hemolítica).
Si recibe tratamiento crónico con medicamentos derivados de plasma humano (como los inhibidores de proteasas) es recomendable que le vacunen frente a la hepatitis A y B.
Se recomienda encarecidamente que cada vez que se le administre Prolastina se anote el nombre y el número de lote del medicamento; ello permite hacer un seguimiento de los pacientes que han recibido cada lote de producto.
Tabaquismo
Dado que la eficacia de Prolastina se ve comprometida por la presencia de humo de tabaco en los pulmones, se recomienda que los pacientes abandonen el hábito de fumar.
Niños y adolescentes
No hay experiencia sobre el uso de Prolastina en niños o adolescentes de menos de 18 años de edad.
Otros medicamentos y Prolastina
No se conocen interacciones entre Prolastina y otros medicamentos.
Comunique a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de usar un medicamento.
No se dispone de ensayos clínicos controlados sobre el uso de este medicamento durante el embarazo. Informe a su médico si está embarazada o cree que pueda estarlo.
Se desconoce si Prolastina pasa a la leche materna, por lo que no debe administrarse a mujeres en periodo de lactancia.
Consulte a su médico si se encuentra en período de lactancia.
Conducción y uso de máquinas
No se han observado efectos sobre la capacidad para conducir o utilizar máquinas.
Prolastina contienesodio
Prolastina 4000 mg contiene aproximadamente 441,6 mg de sodio (componente principal de la sal de mesa/para cocinar).
Prolastina 5000 mg contiene aproximadamente 552,0 mg de sodio (componente principal de la sal de mesa/para cocinar).
En el caso de un paciente de 75 kg de peso corporal la dosis recomendada equivale al 24,84% de la ingesta diaria máxima de sodio recomendada para un adulto. Consulte a su médico o farmacéutico si se le ha recomendado seguir una dieta baja en sal (sodio).
3. Cómo usar Prolastina
Tras reconstituir la solución con el disolvente incluido en el envase, Prolastina debe administrarse por perfusión intravenosa. Un médico especialista en enfermedad pulmonar obstructiva crónica supervisará las primeras perfusiones con Prolastina.
Tratamiento domiciliario
Después de las primeras perfusiones, un profesional sanitario también podría administrar Prolastina, pero únicamente tras haber recibido una formación adecuada. Si su médico decide que usted es apto para dicho tratamiento domiciliario se asegurará de que se proporciona la formación adecuada al profesional sanitario con respecto a:
- cómo preparar y administrar la solución reconstituida para perfusión (ver las instrucciones ilustradas al final de este prospecto,
- cómo mantener el producto estéril (técnicas asépticas de perfusión),
- cómo realizar el registro del tratamiento,
- cómo identificar los efectos adversos, incluidos los síntomas de las reacciones alérgicas y las medidas que deban tomarse en caso de que se manifiesten dichos efectos (ver también la sección 4).
Dosis
La cantidad de Prolastina que se le administra se basa en su peso corporal. Se recomienda administrar una dosis semanal de 60 mg del principio activo por kg de peso corporal (en el caso de un paciente de 75 kg de peso, esta dosis equivale a 180 ml de la solución reconstituida para perfusión y contiene 25 mg de inhibidor de alfa1-antitripsina (humana) por ml), que normalmente es suficiente para mantener los niveles de protección del inhibidor de alfa1-antitripsina para evitar el empeoramiento del enfisema pulmonar.
Su médico le indicará la duración de su tratamiento, ya que hasta la fecha no se ha establecido la necesidad de limitar la duración del tratamiento.
Si tiene la impresión de que el efecto de Prolastina es demasiado fuerte o débil, consulte a su médico o farmacéutico.
Si usa más Prolastina del que debe
Hasta la fecha se desconocen los efectos de una sobredosis.
- Avise a su médico o profesional sanitario si cree que ha usado más Prolastina del que debe para que tome las medidas adecuadas.
- En caso de sobredosis o administración accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad administrada.
Si olvidó usar Prolastina
- Consulte a su médico si la dosis olvidada debería administrarse.
- No administre una dosis doble para compensar la perfusión olvidada.
Si interrumpe el tratamiento con Prolastina
Si se interrumpe el tratamiento con Prolastina, su enfermedad puede empeorar. Consulte a su médico inmediatamente si desea interrumpir el tratamiento con Prolastina.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si los efectos adversos se presentan durante la perfusión de Prolastina, deberá suspender o interrumpir la perfusión, dependiendo de la naturaleza y la gravedad del efecto adverso.
Posibles efectos adversos graves
Raramente pueden presentarse reacciones de hipersensibilidad(pueden afectar hasta una de cada 1.000 personas), en algunos casos muy raros estas reacciones pueden presentarse como reacciones de anafilaxia de cualquier tipo (pueden afectar hasta una de cada 10.000 personas), aunque no se hayan experimentado síntomas de alergia en perfusiones anteriores.
Avise a su médico o enfermero inmediatamentesi observa cualquiera de los siguientes síntomas:
- erupción, sarpullido, picazón,
- dificultad para tragar,
- hinchazón en la cara o la boca,
- enrojecimiento,
- dificultad para respirar (disnea),
- caída de la presión arterial,
- alteración del latido cardíaco,
- escalofrios
Su médico o profesional sanitario decidirá si disminuir o interrumpir la perfusión e iniciar el tratamiento adecuado según sea necesario.
En caso de tratamiento domiciliario interrumpa la perfusión inmediatamentey póngase en contacto con su médico o profesional sanitario.
Durante el tratamiento con Prolastina se han observado los siguientes efectos adversos:
Poco frecuentes (pueden afectar hasta una de cada 100 personas):
- Escalofríos, fiebre, síntomas parecidos a los de la gripe, dolor en el pecho.
- Sarpullido (urticaria).
- Mareo, aturdimiento, dolor de cabeza.
- Dificultad para respirar (disnea).
- Erupciones cutáneas.
- Náuseas.
- Dolor en las articulaciones (artralgia).
Raros (pueden afectar hasta una de cada 1.000 personas):
- Reacciones alérgicas.
- Pulso rápido (taquicardia).
- Tensión arterial baja (hipotensión).
- Tensión arterial alta (hipertensión).
- Dolor de espalda.
Muy raros (pueden afectar hasta una de cada 10.000 personas):
- Shock anafiláctico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Prolastina
No conservar a temperatura superior a 25°C.
No congelar.
No refrigerar la solución después de su reconstitución. La solución reconstituida debe utilizarse siempre en las 3 horas siguientes a su preparación. El producto no utilizado debe desecharse. No tire ningún medicamento por el desagüe ni con la basura doméstica. Pregunte a su farmacéutico cómo puede desechar los medicamentos que ya no utilice. Estas medidas ayudarán a proteger el medioambiente.
Mantener fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del vial y en la caja.
6. Contenido del envase e información adicional
Composición de Prolastina
- El principio activo es alfa1-antitripsina humana (derivada de sangre o plasma humano).
- Los demás componentes son cloruro de sodio, dihidrógeno fosfato de sodio, agua para preparaciones inyectables (disolvente/diluyente).
Aspecto del producto y contenido del envase
Alfa1-antitripsina humana es un polvo de color blanco o amarillo pálido o marrón pálido o una masa friable.
Una vez reconstituida con agua para preparaciones inyectables, la solución debe ser entre transparente y ligeramente opalescente, incolora, verde pálido, amarillo pálido o marrón pálido y libre de partículas visibles.
1 ml de la solución reconstituida contiene 25 mg de alfa1-antitripsina humana.
Un envase individual contiene:
Prolastina 4000 mg polvo y disolvente para solución para perfusión:
- 1 vial con polvo que contiene 4000 mg de alfa1-antitripsina humana.
- 1 vial con 160 ml de disolvente (agua para preparaciones inyectables).
- 1 dispositivo de transferencia para la reconstitución.
Prolastina 5000 mg polvo y disolvente para solución para perfusión:
- 1 vial con polvo que contiene 5000 mg de alfa1-antitripsina humana.
- 1 vial con 200 ml de disolvente (agua para preparaciones inyectables).
- 1 dispositivo de transferencia para la reconstitución.
Titular de la autorización de comercialización y responsable de la fabricación
Titular:
Grifols Deutschland GmbH
Colmarer Straβe 22
60528 Frankfurt
Alemania
Teléfono: +49 69/660 593 100
Email: [email protected]
Fabricante:
Instituto Grifols, S.A.
Can Guasch, 2 – Parets del Vallès
08150 Barcelona
España
Representante Local:
Instituto Grifols, S.A.
Can Guasch, 2 - Parets del Vallès
08150 Barcelona
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Prolastin [4000 mg/5000 mg]: Austria, Irlanda, Italia, Francia, Alemania, Grecia, Holanda, Polonia, Portugal,
Prolastina [4000 mg/5000 mg]: Dinamarca, Finlandia, Noruega, España, Suecia,
Pulmolast [4000 mg/5000 mg]: Bélgica.
Fecha de la última revisión de este prospecto: Septiembre 2023.
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.es/
Esta información está destinada a profesionales sanitarios y a pacientes aptos para tratamiento domiciliario.
Preparación de la solución reconstituida para perfusión:
- Emplee condiciones asépticas (limpieza y desinfección) para mantener la esterilidad. Reconstituya el medicamento en una superficie de trabajo plana durante la preparación de la solución.
- Asegúrese de que los viales de Prolastina en polvo y de disolvente (agua para preparaciones inyectables estéril) están a temperatura ambiente (20-25ºC).
- Retire la cápsula protectora tanto del vial de Prolastina como del vial de disolvente y limpie los bordes del cuello y los tapones con una toallita con alcohol. Deje que los tapones de goma se sequen.
|
|
|
|
|
|
- Si es necesario más de un vial de producto para lograr la dosis requerida, repita las instrucciones anteriores utilizando el envase adicional que contiene un nuevo adaptador de transferencia. No reutilice el adaptador.
La disolución total debe obtenerse en aproximadamente 15 minutos para las presentaciones de 4000 mg y 5000 mg de Prolastina.
Solo deben utilizarse soluciones entre transparentes y ligeramente opalescentes, incoloras, verde pálido, amarillo pálido o marrón pálido y libres de partículas visibles. Prolastina no debe mezclarse con otras soluciones para perfusión. La solución reconstituida debe usarse siempre dentro de las 3 horas después de su preparación.
La solución reconstituida debe administrarse mediante perfusión intravenosa lenta, con un equipo de perfusión (no incluido) adecuado. La velocidad de perfusión debe ser inferior a 0,08 ml por kg de peso corporal por minuto (equivalente a 6 ml por minuto para un paciente de 75 kg de peso).
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a PROLASTINA 5.000 MG POLVO Y DISOLVENTE PARA SOLUCIÓN PARA PERFUSIÓNForma farmacéutica: INYECTABLE PERFUSION, 1000 mgPrincipio activo: alfa1 antitrypsinFabricante: Instituto Grifols S.A.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 1000 mg alfa 1 antitripsinaPrincipio activo: alfa1 antitrypsinFabricante: Grifols Deutschland GmbhRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 4.000 mgPrincipio activo: alfa1 antitrypsinFabricante: Grifols Deutschland GmbhRequiere receta
Médicos online para PROLASTINA 5.000 MG POLVO Y DISOLVENTE PARA SOLUCIÓN PARA PERFUSIÓN
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de PROLASTINA 5.000 MG POLVO Y DISOLVENTE PARA SOLUCIÓN PARA PERFUSIÓN, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






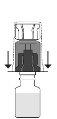 encaje. Evite girarlo.
encaje. Evite girarlo.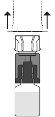
 Empújelo con el terminal transparente/blanco del adaptador recto hacia abajo, sin girarlo, hasta que la punta perfore el tapón y encaje.
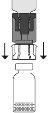
Empújelo con el terminal transparente/blanco del adaptador recto hacia abajo, sin girarlo, hasta que la punta perfore el tapón y encaje.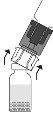 Debido al vacío en el vial de polvo, la transferencia de disolvente se iniciará automáticamente. Espere a que se complete la transferencia del disolvente. Retire el adaptador con el vial de disolvente conectado en un ángulo aproximado de 45°.
Debido al vacío en el vial de polvo, la transferencia de disolvente se iniciará automáticamente. Espere a que se complete la transferencia del disolvente. Retire el adaptador con el vial de disolvente conectado en un ángulo aproximado de 45°. Gire suavemente el vial de Prolastina hasta que el polvo se disuelva completamente. No agite para evitar la formación de espuma. No toque el tapón. Administre el producto con una técnica aséptica.
Gire suavemente el vial de Prolastina hasta que el polvo se disuelva completamente. No agite para evitar la formación de espuma. No toque el tapón. Administre el producto con una técnica aséptica.









