OVITRELLE 250 MICROGRAMOS SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar OVITRELLE 250 MICROGRAMOS SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Ovitrelle 250microgramos solución inyectable en pluma precargada
coriogonadotropina alfa
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Ovitrelle y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ovitrelle
- Cómo usar Ovitrelle
- Posibles efectos adversos
- Conservación de Ovitrelle
- Contenido del envase e información adicional
1. Qué es Ovitrelle y para qué se utiliza
Qué es Ovitrelle
Ovitrelle contiene un medicamento denominado “coriogonadotropina alfa”, fabricado en laboratorio mediante una técnica especial de ADN recombinante. La coriogonadotropina alfa es similar a una hormona que se encuentra en su organismo de forma natural denominada “gonadotropina coriónica”, que interviene en la reproducción y la fertilidad.
Para qué se utiliza Ovitrelle
Ovitrelle se utiliza junto con otros medicamentos:
- Para ayudar a que se desarrollen y maduren varios folículos (cada uno contiene un óvulo) en mujeres sometidas a técnicas de reproducción asistida (procedimiento que puede ayudarle a quedarse embarazada), tales como la “fertilización in vitro”. Se darán primero otros medicamentos para desencadenar el crecimiento de varios folículos.
- Para ayudar a que se libere un óvulo del ovario (inducción de la ovulación) en mujeres que no pueden producir óvulos (“anovulación”) o producen muy pocos (“oligovulación”). Se darán primero otros medicamentos para desarrollar y madurar los folículos.
2. Qué necesita saber antes de empezar a usar Ovitrelle
No use Ovitrelle
- si es alérgica a la coriogonadotropina alfa, o a alguno de los demás componentes de este medicamento (incluidos en la sección 6),
- si tiene un tumor en el hipotálamo o en la hipófisis (ambos son partes del cerebro),
- si tiene ovarios grandes o bolsas grandes de líquido dentro de los ovarios (quistes ováricos) de origen desconocido,
- si tiene hemorragias vaginales de causa desconocida,
- si tiene un cáncer de ovario, útero o mama,
- si padece de inflamación grave de las venas o coágulos de sangre en las venas (problemas tromboembólicos activos),
- si presenta alguna circunstancia que por lo general impide un embarazo normal, como menopausia o menopausia precoz (insuficiencia ovárica), o malformaciones de los órganos sexuales.
No use Ovitrelle si se cumple cualquiera de las anteriores condiciones. Si no está segura, consulte a su médico antes de tomar este medicamento.
Advertencias y precauciones
Antes de iniciar el tratamiento, su fertilidad y la de su pareja deben ser evaluadas por un médico experto en el tratamiento de problemas de fertilidad.
Síndrome de hiperestimulación ovárica (SHO)
Este medicamento puede aumentar el riesgo de que presente un SHO. Esto ocurre cuando los folículos se desarrollan demasiado y se convierten en grandes quistes.
Si nota dolor en la parte inferior del abdomen, aumenta de peso rápidamente, tiene náuseas o vomita, o tiene dificultad para respirar, no se administre la inyección de Ovitrelle y consulte a su médico inmediatamente (ver la sección 4). Si desarrolla un SHO, se le puede indicar que no practique el sexo o que utilice un método anticonceptivo de barrera durante al menos cuatro días.
El riesgo de SHO disminuye si se utiliza la dosis habitual de Ovitrelle y si usted es controlada cuidadosamente a lo largo del ciclo de tratamiento (por ejemplo, mediante análisis de sangre para medir los niveles de estradiol y ecografías).
Embarazo múltiple y/o anomalías congénitas
Durante el uso de Ovitrelle usted tiene un mayor riesgo de quedarse embarazada de más de un bebé al mismo tiempo (“embarazo múltiple”, normalmente de gemelos) que si concibe de forma natural. El embarazo múltiple puede dar lugar a complicaciones para usted y para sus bebés. Durante el tratamiento con técnicas de reproducción asistida, el riesgo de tener un embarazo múltiple está relacionado con su edad y con la calidad y el número de óvulos fertilizados o embriones que se le implanten en el cuerpo. Los embarazos múltiples y ciertas características específicas de las parejas con problemas de fertilidad (p. ej., la edad) pueden estar relacionados también con un aumento de las probabilidades de anomalías congénitas.
El riesgo de embarazos múltiples disminuye si usted es controlada cuidadosamente a lo largo del ciclo de tratamiento (por ejemplo, mediante análisis de sangre para medir los niveles de estradiol y ecografías).
Embarazo ectópico
Puede producirse un embarazo fuera del útero (embarazo ectópico) en mujeres con lesiones en las trompas de Falopio (los conductos que transportan el óvulo desde el ovario hasta el útero). Por tanto, su médico debe realizar un examen ecográfico temprano para descartar la posibilidad de embarazo fuera del útero.
Aborto
Durante el tratamiento con técnicas de reproducción asistida o de estimulación de sus ovarios para producir óvulos, tiene una mayor probabilidad de tener un aborto que la media de las mujeres.
Problemas de coagulación de la sangre (episodios tromboembólicos)
Consulte a su médico antes de usar Ovitrelle si usted o algún familiar han tenido alguna vez coágulos sanguíneos en la pierna o en el pulmón, o un ataque al corazón o un ictus. Puede correr un riesgo mayor de coágulos sanguíneos graves o de que coágulos existentes se agraven al recibir tratamiento con Ovitrelle.
Tumores de los órganos sexuales
Se han comunicado tumores en los ovarios y en otros órganos sexuales, tanto benignos como malignos, en mujeres que han recibido diversos tratamientos farmacológicos para la infertilidad.
Pruebas de embarazo
Si se realiza una prueba de embarazo con suero u orina después de usar Ovitrelle, y hasta diez días después, puede ocurrir que obtenga un resultado de la prueba falso positivo. Si no está segura, consúltelo con su médico.
Niños y adolescentes
Ovitrelle no se debe utilizar en niños y adolescentes.
Otros medicamentos y Ovitrelle
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
No use Ovitrelle si está embarazada o en periodo de lactancia.
Si está embarazada o en periodo de lactancia, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
No se prevé que Ovitrelle afecte a su capacidad de conducir o usar máquinas.
Ovitrelle contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Ovitrelle
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cuánto utilizar
- La dosis recomendada de Ovitrelle es 1 pluma precargada (250 microgramos/0,5 ml) en una inyección única.
- Su médico le explicará exactamente cuándo debe ponerse la inyección.
Uso de este medicamento
- Si se va a administrar Ovitrelle usted misma, lea atentamente y siga las “Instrucciones de uso”.
- Ovitrelle se administra mediante inyección bajo la piel (subcutáneamente).
- Cada pluma precargada es para un solo uso.
- Su médico o enfermero le enseñará cómo usar la pluma precargada de Ovitrelle para inyectar el medicamento.
- Inyéctese Ovitrelle del modo que le enseñó su médico o enfermero.
- Después de la inyección, deseche la aguja usada de forma segura y deseche la pluma.
Si usa más Ovitrelle del que debe
Los efectos de una sobredosis de Ovitrelle son desconocidos; sin embargo, existe la posibilidad de que se produzca un síndrome de hiperestimulación ovárica (SHO), que se describe más ampliamente en la sección 4.
Si olvidó usar Ovitrelle
Si olvidó usar Ovitrelle, contacte con su médico tan pronto como se dé cuenta.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si nota alguno de los siguientes efectos adversos graves, interrumpa el uso de Ovitrelle y consulte inmediatamente a un médico, puede que necesite tratamiento médico urgente:
- Las reacciones alérgicas tales como erupción, pulso acelerado o irregular, hinchazón de la lengua o garganta, estornudos, sibilancias o dificultad respiratoria grave son muy raras (pueden afectar hasta a 1 de cada 10.000 personas).
- El dolor en la parte inferior del abdomen, la distensión abdominal o las molestias abdominales acompañados de náuseas (tener ganas de vomitar) o los vómitos pueden ser síntomas del síndrome de hiperestimulación ovárica (SHO). Esto puede indicar que los ovarios reaccionaron de manera exagerada al tratamiento y se desarrollaron grandes quistes ováricos (ver también en la sección 2 bajo “Síndrome de hiperestimulación ovárica”). Estos episodios son frecuentes (pueden afectar hasta a 1 de cada 10 personas).
- El SHO puede llegar a hacerse grave con ovarios claramente agrandados, un descenso en la producción de orina, aumento de peso, dificultad respiratoria y posible acumulación de líquidos en el estómago o pecho. Estos episodios son poco frecuentes (pueden afectar hasta a 1 de cada 100 personas).
- Las complicaciones graves de coagulación de la sangre (episodios tromboembólicos), a veces independientes del SHO, se observan muy raramente. Éstas podrían provocar dolor en el pecho, falta de aliento, ictus o ataque al corazón (ver también en la sección 2 bajo “Problemas de coagulación de la sangre”).
Otros efectos adversos
Frecuentes (pueden afectar hasta a 1 de cada 10 personas)
- Dolor de cabeza.
- Reacciones locales en el lugar de inyección, tales como dolor, enrojecimiento o hinchazón.
Poco frecuentes (pueden afectar hasta a 1 de cada 100 personas)
- Diarrea.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ovitrelle
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC). No congelar.
No utilice Ovitrelle si observa indicios visibles de deterioro, si el líquido contiene partículas o no es transparente.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ovitrelle
- El principio activo es coriogonadotropina alfa, producida por tecnología de ADN recombinante.
- Cada pluma precargada contiene 250 microgramos de coriogonadotropina alfa en 0,5 ml (equivalente a aproximadamente 6.500 Unidades Internacionales, UI).
- Los demás componentes son manitol, metionina, hidrogenofosfato de disodio dihidrato, dihidrogenofosfato de sodio monohidrato, poloxámero 188, ácido fosfórico (para el ajuste del pH), hidróxido sódico (para el ajuste del pH) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
- Ovitrelle se presenta como un líquido transparente, incoloro o ligeramente amarillento para inyección en una pluma precargada.
- Cada pluma contiene 0,5 ml de solución.
- Se suministra en envases de 1 pluma precargada y 2 agujas para inyección (una de repuesto).
Titular de la autorización de comercialización
Merck Europe B.V., Gustav Mahlerplein 102, 1082 MA Amsterdam, Países Bajos
Responsable de la fabricación
Merck Serono S.p.A., Via delle Magnolie 15, 70026 Modugno (Bari), Italia.
Fecha de la última revisión de este prospecto:05/2025.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
INSTRUCCIONES DE USO
Ovitrelle 250microgramos
Solución inyectable en pluma precargada
Coriogonadotropina alfa
Información importante sobre la pluma precargada de Ovitrelle
- Lea las instrucciones de uso y el prospecto antes de usar la pluma precargada de Ovitrelle.
- Siga siempre todas las indicaciones de estas instrucciones de uso y la formación que le ha proporcionado el profesional sanitario, ya que pueden ser distintas de las recibidas con anterioridad. Esta información permitirá evitar errores en el tratamiento o infecciones por pinchazo de aguja o lesiones por rotura del vidrio.
- La pluma precargada de Ovitrelle es solo para inyección por vía subcutánea.
- La pluma precargada de Ovitrelle es para un solo uso.
- Cada envase de pluma precargada de Ovitrelle contiene una aguja para la inyección y una aguja de reserva.
- Solo use la pluma precargada de Ovitrelle si el profesional sanitario le enseña cómo usarla correctamente.
- Conservar en nevera.
Nocongelar.
Nocomparta la pluma ni las agujas con ninguna otra persona.
Noutilice la pluma precargada de Ovitrelle si se ha caído, o si la pluma está agrietada o dañada, ya que esto puede causar lesiones.
Familiarícese con la pluma precargada de Ovitrelle
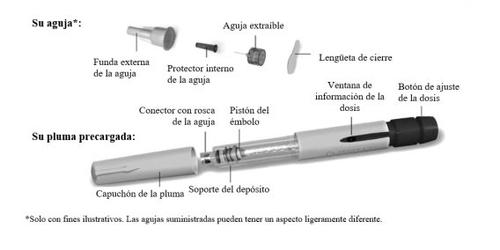
Paso1 Reúna los materiales
1.1Prepare un lugar limpio y una superficie plana, como una mesa o encimera, en una zona bien iluminada. |
|
1.2También necesitará (no incluidos en el envase):
| |
1.3Lávese las manos con agua y jabón y séquelas bien a continuación (Figura 2). | |
1.4Extraiga con la mano la pluma precargada de Ovitrelle del envase. | |
Noutilice ningún utensilio, ya que su uso puede dañar la pluma. | |
1.5Compruebe que sobre la pluma precargada ponga Ovitrelle. 1.6Compruebe la fecha de caducidad en la etiqueta de la pluma (Figura 3). | |
Noutilice la pluma precargada de Ovitrelle si ya ha pasado la fecha de caducidad o si en la pluma precargada no pone Ovitrelle. |
Paso2 Prepárese para la inyección
2.1Retire el capuchón de la pluma (Figura 4). 2.2Compruebe que el medicamento es transparente y entre incoloro y amarillento y que no contiene partículas. Noutilice la pluma precargada si el medicamento ha cambiado de color o está turbio, ya que esto puede causar una infección. |
|
Elija un lugar de inyección: | |
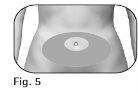
2.3El profesional sanitario deberá indicarle los lugares de inyección que debe utilizar alrededor de la zona del estómago (Figura 5). 2.4Limpie la piel del lugar de inyección con una toallita empapada en alcohol. Notoque ni cubra la piel que acaba de limpiar. |
|
Paso3 Acople la aguja
3.1Tome una nueva aguja. Utilice solamente las agujas de un solo uso suministradas. 3.2Compruebe que la funda externa de la aguja no esté dañada. 3.3Sujete con firmeza la funda externa de la aguja. |
|
3.4Compruebe que la lengüeta de cierre de la funda externa de la aguja no esté dañada o suelta y que no ha pasado la fecha de caducidad (Figura 6). |
|
3.5Retire la lengüeta de cierre (Figura 7). |
Noutilice la aguja si está dañada o caducada o si la funda externa de la aguja o la lengüeta de cierre está dañada o suelta. Utilizar agujas caducadas o agujas con lengüetas de cierre o fundas externas de la aguja dañadas puede causar una infección. Tírela en un contenedor para objetos cortantes y punzantes y utilice la otra aguja suministrada en el envase.
Pregunte a su profesional sanitario si tiene alguna duda.
3.6Enrosque la funda externa de la aguja en el conector roscado de la aguja de la pluma precargada de Ovitrelle hasta que note una ligera resistencia (Figura 8). Noapriete demasiado la aguja al acoplarla, ya que podría ser difícil extraerla después de la inyección. |
|
3.7Retire la funda externa de la aguja tirando de ella suavemente (Figura 9). 3.8Déjela a un lado para usarla después (Figura 10). |
|
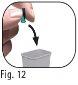
Nodeseche la funda externa de la aguja, ya que esta evitará lesiones por pinchazo con la aguja e infecciones al separar la aguja de la pluma precargada. 3.9Sostenga la pluma precargada de Ovitrelle con la aguja apuntando hacia arriba (Figura 11). 3.10Retire cuidadosamente y deseche el protector interno de la aguja (Figura 12). |
|
Novuelva a tapar la aguja con el protector interno de la aguja, ya que esto puede provocar lesiones por pinchazo con la aguja e infecciones.
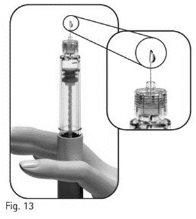
3.11Examine minuciosamente la punta de la aguja en busca de una o varias gotitas de líquido (Figura 13).
|
|
Si no observa ninguna gotita de líquido en la punta o sus proximidades cuando utilice una pluma nueva:
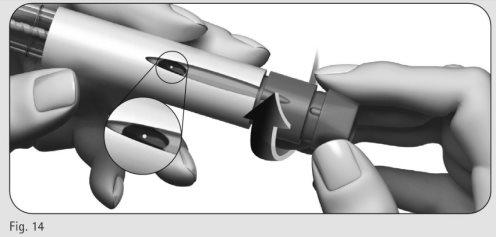
- Gire con cuidado el botón de ajuste de la dosis hacia delante hasta que vea un punto (?) en la ventana de información de la dosis (Figura 14).
- Puede girar el botón de ajuste de la dosis hacia atrás si lo ha desplazado más allá del punto (?).

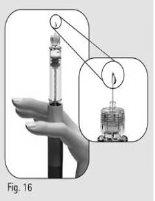

- Sostenga la pluma con la aguja apuntando hacia arriba.
- Golpee suavemente el soporte del depósito (Figura 15).
- Pulse el botón de ajuste de la dosis por completo. Aparecerá una gotita de líquido en la punta de la aguja (Figura 16)*.
- Compruebe que la ventana de información de la dosis indica “0” (Figura 17).
*Nota: Si no observa ningún líquido, puede empezar de nuevo en el paso 1 (en esta sección) una vez más únicamente. Si tampoco aparece una gotita de líquido la segunda vez, póngase en contacto con el profesional sanitario.
Paso4 Seleccione la dosis de 250
4.1Gire suavemente el botón de ajuste de la dosis hacia delantehasta que aparezca “250”en la ventana de información de la dosis. | |
Nopresione ni tire del botón de ajuste de la dosis mientras lo gira. |
|
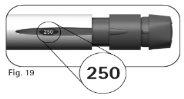
4.2Compruebe que la ventana de información de la dosisindica “250”(Figura 19) antes de continuar con el paso 5 más abajo. Póngase en contacto con el profesional sanitario si necesita ayuda. |
|
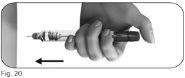
Paso5 Inyecte la dosis
Importante:inyecte la dosis tal como le ha enseñado el profesional sanitario.
5.1Inserte lentamente la totalidad de la aguja en la piel (Figura 20). |
|
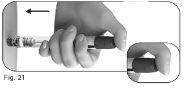
5.2Coloque el pulgar en el centro del botón de ajuste de la dosis. Apriete el botón de ajuste de la dosis lenta y completamentey manténgalo apretado para administrar íntegramente la inyección (Figura 21). |
|
5.3Mantenga el botón de ajuste de la dosis apretado durante un mínimo de 5 segundos antes de extraer la aguja de la piel (Figura 22).
|
|
|
Nosuelte el botón de ajuste de la dosis hasta haber retirado la aguja de la piel.
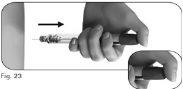
Paso6 Retire la aguja después de la inyección
6.1Coloque la funda externa de la aguja sobre una superficie plana. |
|
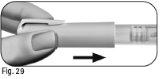
6.2Sostenga la pluma precargada de Ovitrelle firmemente con una mano e introduzca la aguja en la funda externa de la aguja (Figura 24). 6.3Continúe empujando la aguja enfundada contra una superficie firme hasta que oiga un chasquido (“clic”) (Figura 25). 6.4Sujete la funda externa de la aguja y desenrosque la aguja girándola en la dirección opuesta (Figura 26). 6.5Deseche la aguja usada de forma segura en un contenedor para objetos cortantes y punzantes (Figura 27). Manipule la aguja con cuidado para evitar lesionarse con ella. Noreutilice ni comparta ninguna aguja usada con ninguna otra persona. |
|
Paso7 Después de la inyección
- Compruebe que se ha administrado una inyección completa:
|
|
Si la ventana de información de la dosis muestra “0”, ha completado la dosis.
Si la ventana de información de la dosis nomuestra “0”, póngase en contacto con el profesional sanitario.
Nointente administrar la inyección una segunda vez.
Paso8 Deseche la pluma precargada de Ovitrelle
Importante:La pluma precargada de Ovitrelle y las agujas suministradas son para un solo uso.
|
Póngase en contacto con el profesional sanitario si tiene alguna pregunta.
Fecha de la última revisión de estas instrucciones de uso: 05/2025.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a OVITRELLE 250 MICROGRAMOS SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 150 UI/ 0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: INYECTABLE, 150 UI/ 0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: INYECTABLE, 150UI/0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere receta
Médicos online para OVITRELLE 250 MICROGRAMOS SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de OVITRELLE 250 MICROGRAMOS SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes