
OTULFI 45 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar OTULFI 45 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Otulfi 45mg solución inyectable en jeringa precargada
ustekinumab
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
Este prospecto ha sido redactado para la persona que hace uso del medicamento. Si usted es el padre o cuidador de un niño al que le administrará Otulfi, por favor, lea atentamente esta información.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque presenten los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Otulfi y para qué se utiliza
- Qué necesita saber antes de empezar a usar Otulfi
- Cómo usar Otulfi
- Posibles efectos adversos
- Conservación de Otulfi
- Contenido del envase e información adicional
1. Qué es Otulfi y para qué se utiliza
Qué es Otulfi
Otulfi contiene el principio activo “ustekinumab”, un anticuerpo monoclonal. Los anticuerpos monoclonales son proteínas que identifican y se unen específicamente a ciertas proteínas del cuerpo.
Otulfi pertenece a un grupo de medicamentos llamados “inmunosupresores”. Estos medicamentos actúan debilitando parte del sistema inmune.
Para qué se utiliza Otulfi
Otulfi se utiliza para el tratamiento de las siguientes enfermedades inflamatorias:
- Psoriasis en placas - en adultos y niños de 6 años en adelante
- Artritis psoriásica - en adultos
- Enfermedad de Crohn de moderada a grave - en adultos
Psoriasis en placas
La psoriasis en placas es una enfermedad de la piel que causa inflamación afectando a la piel y las uñas. Otulfi reduce la inflamación y otros signos de la enfermedad.
Otulfi se utiliza en adultos con psoriasis en placas de moderada a grave, que no pueden utilizar ciclosporina, metotrexato o fototerapia, o donde estos tratamientos no funcionan.
Otulfi se utiliza en niños y adolescentes de 6 años de edad en adelante con psoriasis en placas de moderada a grave que no son capaces de tolerar la fototerapia u otras terapias sistémicas o cuando estos tratamientos no funcionan.
Artritis psoriásica
La artritis psoriásica es una enfermedad inflamatoria de las articulaciones, que normalmente va acompañada de psoriasis. Si tiene artritis psoriásica activa, primero recibirá otros medicamentos. Si no responde bien a estos medicamentos, puede ser tratado con Otulfi para:
- Reducir los signos y síntomas de su enfermedad.
- Mejorar su función física.
- Reducir el daño en sus articulaciones.
Enfermedad de Crohn
La enfermedad de Crohn es una enfermedad inflamatoria del intestino. Si padece la enfermedad de Crohn, le administrarán primero otros medicamentos. Si no responde de manera adecuada o no tolera esos medicamentos, puede que le administren Otulfi para reducir los signos y síntomas de su enfermedad.
2. Qué necesita saber antes de empezar a usar Otulfi
No use Otulfi
- Si es alérgico a ustekinumabo a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si tiene una infección activaque su médico piense que es importante.
Si no está seguro si algunode los puntos anteriores le concierne, hable con su médico o farmacéutico antes de usar Otulfi.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Otulfi. Su médico comprobará cómo se encuentra antes de cada tratamiento. Asegúrese de informar a su médico sobre cualquier enfermedad que sufra antes de cada tratamiento. También su médico le preguntará si recientemente ha estado cerca de alguien que pudiera tener tuberculosis. Su médico le examinará y le hará un test para detección de la tuberculosis, antes de usar Otulfi. Si su médico cree que usted está en riesgo de tuberculosis, puede darle medicamentos para tratarla.
Observe los efectos adversos graves
Otulfi puede causar efectos adversos graves, incluyendo reacciones alérgicas e infecciones. Usted debe prestar atención a ciertos signos de enfermedad mientras esté utilizando Otulfi. Ver la lista completa de estos efectos adversos en “Efectos adversos graves” de la sección 4.
Antes de utilizar Otulfi dígale a su médico:
- Si usted ha tenido alguna vez una reacción alérgica a ustekinumab.Consulte con su médico si no está seguro.
- Si usted alguna vez ha tenido algún tipo de cáncer– esto es porque los inmunosupresores del tipo de Otulfi debilitan parte del sistema inmunitario. Esto puede aumentar el riesgo de tener cáncer.
- Si usted ha recibido tratamiento para la psoriasis con otros biológicos (un medicamento producido a partir de una fuente biológica y que suele administrarse mediante inyección)– el riesgo de padecer cáncer puede ser mayor.
- Si tiene o ha tenido una infección reciente.
- Si tiene cualquier lesión nueva o cambio de las lesionesdentro del área de psoriasis o sobre la piel intacta.
- Si usted alguna vez ha tenido una reacción alérgica a la inyección de Otulfi. Ver “Observe los efectos adversos graves” en la sección 4 para los signos de una reacción alérgica.
- Si usted está tomando cualquier otro tratamiento para la psoriasis y/o artritis psoriásica– como cualquier otro inmunosupresor o fototerapia (cuando su cuerpo es tratado con un tipo de luz ultravioleta (UV)). Estos tratamientos pueden también debilitar parte del sistema inmunitario. No se ha estudiado el uso de estos tratamientos de manera conjunta con Otulfi. Sin embargo, es posible que pueda aumentar la probabilidad de sufrir enfermedades relacionadas con un sistema inmune más débil.
- Si usted está recibiendo o ha recibido alguna vez inyecciones para tratar las alergias– se desconoce si Otulfi puede afectar a estos tratamientos.
- Si usted tiene 65años o más– usted tiene más probabilidades de adquirir infecciones.
Si no está seguro de no padecer alguno de estos trastornos, hable con su médico o farmacéutico antes de usar Otulfi.
Algunos pacientes han experimentado reacciones similares al lupus durante el tratamiento con ustekinumab, incluido lupus cutáneo o síndrome tipo lupus. Hable con su médico de inmediato si experimenta erupción cutánea roja, elevada y escamosa, a veces con un borde más oscuro, en zonas de la piel expuestas al sol o si van acompañadas de dolores articulares.
Ataques al corazón e ictus
En un estudio realizado en pacientes con psoriasis tratados con ustekinumab se han observado ataques al corazón e ictus. Su médico comprobará periódicamente sus factores de riesgo de enfermedad cardíaca e ictus para garantizar que se tratan adecuadamente. Busque atención médica de inmediato si presenta dolor torácico, debilidad o sensación anormal en un lado del cuerpo, parálisis facial o anomalías en el habla o la vista.
Niños y adolescentes
No se recomienda el uso de Otulfi en niños menores de 6 años de edad con psoriasis ni en niños menores de 18 años de edad con artritis psoriásica y enfermedad de Crohn, ya que no ha sido estudiado en este grupo de edad.
Uso de Otulfi con otros medicamentos, vacunas
Informe a su médico o farmacéutico:
- Si está utilizando, ha utilizado recientemente o puede utilizar otros medicamentos.
- Si ha sido vacunado recientemente o va a recibir una vacuna. No se deben administrar determinados tipos de vacunas (vacunas vivas) mientras se utilice Otulfi.
- Si recibió Otulfi durante el embarazo, informe al médico de su lactante sobre su tratamiento con Otulfi antes de que el lactante reciba cualquier vacuna, incluidas las vacunas vivas, como la vacuna BCG (utilizada para prevenir la tuberculosis). No se recomiendan las vacunas vivas para su lactante en los primeros doce meses después del nacimiento si usted recibió Otulfi durante el embarazo, a menos que el médico de su lactante recomiende lo contrario.
Embarazo y lactancia
- Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
- No se ha observado un mayor riesgo de defectos de nacimiento en bebés expuestos a ustekinumab en el útero. Sin embargo, existe experiencia limitada con ustekinumab en mujeres embarazadas. Por tanto, es preferible evitar el uso de Otulfi durante el embarazo.
- Si es una mujer en edad fértil, se le recomienda que evite quedarse embarazada y use medidas anticonceptivas adecuadas mientras esté utilizando Otulfi y durante al menos 15 semanas tras el último tratamiento con Otulfi.
- Ustekinumab puede pasar a través de la placenta al feto. Si recibió Otulfi durante el embarazo, su lactante podría tener un mayor riesgo de contraer una infección.
- Es importante que informe a los médicos de su lactante y a otros profesionales de la salud si recibió Otulfi durante su embarazo antes de que el lactante reciba cualquier vacuna. No se recomiendan las vacunas vivas, como la vacuna BCG (utilizada para prevenir la tuberculosis) para su lactante en los primeros doce meses después del nacimiento si usted recibió Otulfi durante el embarazo, a menos que el médico de su lactante recomiende lo contrario.
- Ustekinumab puede excretarse en la leche materna en cantidades muy pequeñas. Informe a su médico si está dando el pecho o tiene previsto hacerlo. Usted y su médico decidirán si debe dar el pecho o utilizar Otulfi. No haga ambas cosas a la vez.
Conducción y uso de máquinas
La influencia de Otulfi sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
Otulfi contiene sodio
Otulfi contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”. No obstante, antes de que se le administrar Otulfi, se mezcla con una solución que contiene sodio. Hable con su médico si sigue una dieta baja en sal.
Otulfi contiene polisorbatos
Este medicamento contiene 0,02 mg de polisorbato 80 en cada jeringa precargada, lo que equivale a 0,04 mg/ml. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene alguna alergia conocida.
3. Cómo usar Otulfi
Otulfi se debe utilizar bajo la guía y supervisión de un médico con experiencia en el tratamiento de las afecciones para las que está indicado Otulfi.
Siempre siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, pregunte a su médico. Pregunte a su médico cuándo deben ponerle las inyecciones y sobre las consultas de seguimiento.
Qué cantidad de Otulfi se administra
Su médico decidirá la cantidad de Otulfi que necesita utilizar y la duración del tratamiento.
Adultos a partir de 18años de edad
Psoriasis o artritis psoriásica
- La dosis recomendada de inicio es de 45 mg de Otulfi. Los pacientes que pesen más de 100 kilogramos (kg) pueden empezar con una dosis de 90 mg en lugar de 45 mg.
- Tras la dosis inicial, tomará la siguiente dosis 4 semanas después y posteriormente, cada 12 semanas. Las dosis siguientes, normalmente son las mismas que la dosis de inicio.
Enfermedad de Crohn
- Durante el tratamiento, el médico le administrará la primera dosis de aproximadamente 6 mg/kg de Otulfi mediante goteo en una vena del brazo (perfusión intravenosa). Después de la dosis inicial, recibirá la siguiente dosis de 90 mg de Otulfi al cabo de 8 semanas y, a partir de entonces, cada 12 semanas, mediante una inyección bajo la piel (“por vía subcutánea”).
- En algunos pacientes, después de la primera inyección bajo la piel, se administrarán 90 mg de Otulfi cada 8 semanas. Su médico decidirá cuándo debe recibir la dosis siguiente.
Niños y adolescentes de 6años de edad en adelante
Psoriasis
- El médico le indicará la dosis correcta para usted, incluyendo la cantidad (volumen) de Otulfi a inyectar para dar la dosis correcta. La dosis adecuada para usted dependerá de su peso corporal en el momento en el que se da cada dosis.
- Si usted pesa entre 60 kg y 100 kg, la dosis recomendada es de 45 mg de Otulfi.
- Si usted pesa más de 100 kg, la dosis recomendada es de 90 mg de Otulfi.
- Tras la dosis inicial, recibirá la siguiente dosis 4 semanas más tarde, y posteriormente cada 12 semanas.
Cómo se administra Otulfi
- Otulfi se administra mediante inyección bajo la piel (“por vía subcutánea”). Al principio de su tratamiento, el personal médico o de enfermería pueden inyectarle Otulfi.
- Sin embargo, usted y su médico pueden decidir que se inyecte Otulfi usted mismo. En ese caso, será entrenado en cómo inyectarse Otulfi usted mismo. En los niños de 6 años de edad en adelante, se recomienda que la administración de Otulfi la realice un profesional sanitario o un cuidador tras recibir el entrenamiento adecuado.
- Para las instrucciones sobre como inyectar Otulfi, ver “Instrucciones de administración” al final de este prospecto.
Consulte con su médico si tiene cualquier pregunta sobre cómo autoinyectarse.
Si usa más Otulfi del que debe
Si ha usado o le han administrado demasiado Otulfi, hable enseguida con su médico o farmacéutico. Lleve siempre consigo la caja del medicamento, aunque esté vacía.
Si olvidó usar Otulfi
Si olvida una dosis, hable con su médico o farmacéutico. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Otulfi
Dejar de usar Otulfi no es peligroso. Sin embargo, si usted lo interrumpe, sus síntomas pueden volver a aparecer.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Algunos pacientes podrían tener efectos adversos graves que pueden necesitar tratamiento urgente.
Reacciones alérgicas – éstas pueden necesitar tratamiento urgente. Contacte con su médico o consiga ayuda médica de urgencia inmediatamente si nota cualquiera de los siguientes signos.
- Las reacciones alérgicas graves (“anafilaxia”) son raras en la población que utiliza ustekinumab (pueden afectar hasta 1 de cada 1.000 personas). Los signos incluyen:
- dificultad para respirar y tragar
- tensión arterial baja, que puede causar mareos o ligeros dolores de cabeza
- hinchazón de la cara, labios, boca o garganta.
- Los signos comunes de una reacción alérgica incluyen erupción cutánea y urticaria (éstos pueden afectar hasta 1 de cada 100 personas).
En casos raros, se han notificado reacciones alérgicas a nivel del pulmón e inflamación del pulmón en pacientes tratados con ustekinumab. Informe a su médico de forma inmediata si tiene síntomas como tos, dificultad para respirar y fiebre.
Si tiene una reacción alérgica grave, su médico puede decidir que usted no debe utilizar Otulfi de nuevo.
Infecciones – éstas pueden necesitar tratamiento urgente. Contacte inmediatamente con su médico si nota cualquiera de estos signos.
- Las infecciones de nariz o garganta y el resfriado común son frecuentes (pueden afectar hasta 1 de cada 10 personas).
- Las infecciones del pecho son poco frecuentes (pueden afectar hasta 1 de cada 100 personas).
- La inflamación de los tejidos situados bajo la piel (“celulitis”) es poco frecuente (puede afectar hasta 1 de cada 100 personas).
- Los Herpes (un tipo de erupción dolorosa con ampollas) son poco frecuentes (pueden afectar hasta 1 de cada 100 personas).
Otulfi puede afectar a su capacidad para combatir infecciones. Algunas de ellas podrían llegar a ser graves y estar causadas por virus, hongos, bacterias (incluida la tuberculosis) o parásitos, y entre ellas se incluyen las infecciones que se producen principalmente en personas con un sistema inmunitario debilitado (infecciones oportunistas). Se han notificado infecciones oportunistas del cerebro (encefalitis, meningitis), los pulmones y los ojos en pacientes que reciben tratamiento con ustekinumab.
Debe vigilar los signos de infección mientras esté usando Otulfi. Éstos incluyen:
- fiebre, síntomas gripales, sudores nocturnos, pérdida de peso
- sensación de cansancio o dificultad para respirar; tos que no desaparece
- tener la piel caliente, enrojecida y dolorosa o tener una erupción dolorosa de la piel con ampollas
- escozor al orinar
- diarrea
- deterioro visual o pérdida de la visión
- cefalea, contractura de la nuca, fotosensibilidad, náuseas o confusión.
Comuníquese con su médico inmediatamente si usted nota cualquiera de estos signos de infección, ya que pueden ser signos de infecciones como las infecciones del pecho, infecciones de la piel, herpes o infecciones oportunistas que podrían tener complicaciones graves. También debe comunicar a su médico si tiene cualquier tipo de infección que no desaparezca o reaparezca. Su médico puede decidir que usted no debe usar Otulfi hasta que la infección desaparezca. También contacte con su médico si tiene algún corte abierto o úlcera que pueda infectarse.
Desprendimiento de la piel – el aumento del enrojecimiento y el desprendimiento de la piel en una superficie amplia del cuerpo pueden ser síntomas de psoriasis eritrodérmica o dermatitis exfoliativa, que son trastornos graves de la piel. Si nota alguno de estos síntomas, debe comunicárselo a su médico inmediatamente.
Otros efectos adversos
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas):
- Diarrea
- Náuseas
- Vómitos
- Sensación de cansancio
- Sensación de mareo
- Dolor de cabeza
- Picor (“prurito”)
- Dolor de espalda, muscular o articular
- Dolor de garganta
- Enrojecimiento y dolor en el lugar de inyección
- Sinusitis
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- Infecciones dentales
- Infecciones vaginales por levaduras
- Depresión
- Taponamiento o congestión nasal
- Hemorragia, cardenales, endurecimiento, hinchazón y picor en el lugar de la inyección
- Sentirse débil
- Párpado caído y hundimiento de los músculos de un lado de la cara (“parálisis facial” o “parálisis de Bell”), que es normalmente temporal
- Un cambio en la psoriasis con enrojecimiento y con nueva ampolla de la piel pequeña, amarilla o blanca, algunas veces acompañada de fiebre (psoriasis pustular)
- Descamación de la piel (exfoliación de la piel)
- Acné
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas):
- Enrojecimiento y desprendimiento de la piel en una superficie amplia del cuerpo, que puede producir picor o dolor (dermatitis exfoliativa). Pueden desarrollarse síntomas similares como un cambio natural de los síntomas de la psoriasis (psoriasis eritrodérmica)
- Inflamación de pequeños vasos sanguíneos, que puede producir una erupción de la piel con pequeños abultamientos de color rojo o púrpura, fiebre o dolor articular (vasculitis)
Efectos adversos muy raros(pueden afectar hasta 1 de cada 10.000 personas)
- Ampollas en la piel, que pueden ser rojas y producir picor y dolor (penfigoide ampolloso).
- Lupus cutáneo o síndrome tipo lupus (erupción cutánea roja, elevada y escamosa en zonas de la piel expuestas al sol, posiblemente acompañado de dolores articulares).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Otulfi
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- Conservar en nevera (2 °C y 8 °C). No congelar.
- Conservar la jeringa precargada en el embalaje exterior para protegerla de la luz.
- Si fuera necesario, las jeringas precargadas individuales de Otulfi se pueden también conservar a temperatura ambiente hasta 30°C durante como máximo un único periodo de tiempo de hasta 30 días en su caja original con el fin de protegerlas de la luz. Escriba la fecha cuando la jeringa precargada se retira por primera vez de la nevera y la fecha cuando se tiene que desechar en los espacios previstos del embalaje exterior. La fecha de desecho no debe exceder la fecha de caducidad original impresa en la caja. Una vez que una jeringa se ha conservado a temperatura ambiente (hasta como máximo 30°C), no se debe guardar de nuevo en la nevera. Deseche la jeringa si no se utiliza dentro de los 30 días de conservación a temperatura ambiente o a partir de la fecha de caducidad original, cualquiera de las dos que ocurra antes.
- No agite las jeringas precargadas de Otulfi. La agitación enérgica prolongada puede deteriorar el producto.
No utilice este medicamento:
- Después de la fecha de caducidad que aparece en la etiqueta y el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
- Si el líquido cambia de color, está turbio o presenta partículas extrañas flotando en él (vea la sección 6 “Aspecto de Otulfi y contenido del envase”).
- Si sabe o cree que ha estado expuesto a temperaturas extremas (como un calentamiento o una congelación accidental).
- Si el producto se ha agitado enérgicamente.
Otulfi es para un único uso. Debe tirar el producto sin usar que quede en la jeringa. Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Otulfi
- El principio activo es ustekinumab. Cada jeringa precargada contiene 45 mg de ustekinumab en 0,5 ml.
- Los demás componentes son L‑histidina, polisorbato 80, sacarosa, agua para preparaciones inyectables y ácido clorhídrico (para ajuste del pH).
Aspecto de Otulfi y contenido del envase
Otulfi es una solución inyectable transparente, entre incolora y de color ligeramente pardo‑amarillo.
Se presenta en un envase que contiene 1 jeringa precargada de 1 ml de vidrio unidosis. Cada jeringa precargada contiene 45 mg de ustekinumab en 0,5 ml de solución inyectable.
Titular de la Autorización de Comercialización
Fresenius Kabi Deutschland GmbH
Else‑Kroener‑Strasse 1
61352 Bad Homburg v.d.Hoehe
Alemania
Responsable de la fabricación
Fresenius Kabi Austria GmbH
Hafnerstraße 36
8055 Graz Austria
Fecha de la última revisión de este prospecto
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu/..
Instrucciones de administración
Al inicio del tratamiento, el profesional sanitario le ayudará con su primera inyección. Sin embargo, es posible que usted y su médico decidan que usted mismo puede inyectarse Otulfi. En tal caso, le enseñarán la manera de inyectarse Otulfi. Hable con su médico si tiene alguna duda sobre la administración de las inyecciones. En los niños de 6 años de edad en adelante, se recomienda que la administración de Otulfi la realice un profesional sanitario o un cuidador tras recibir el entrenamiento adecuado.
- No mezcle Otulfi con otros líquidos inyectables.
- No agite las jeringas precargadas de Otulfi. El medicamento puede deteriorarse si se agita con energía. No use el medicamento si se ha agitado enérgicamente.
La Figura 1 muestra cómo es la jeringa precargada.
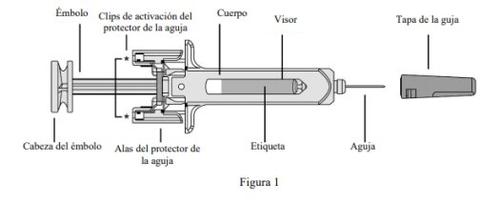
- Compruebe el número de jeringas precargadas y prepare los materiales: Preparación para utilizar la jeringa precargada
- Saque la(s) jeringa(s) precargada(s) de la nevera. Deje la jeringa precargada fuera de la caja durante 30 minutos. Esto permitirá que el líquido alcance una temperatura agradable para su administración (temperatura ambiente). No retire la tapa de la aguja mientras espera a que se alcance la temperatura ambiente.
- Sujete la jeringa precargada por el cuerpo con la aguja tapada apuntando hacia arriba.
- No coja la jeringa por la cabeza del émbolo, el émbolo, las alas del protector de la aguja o la tapa de la aguja.
- No retire el émbolo en ningún momento.
- No retire la tapa de la jeringa precargada hasta que se le indique.
- No toque los clips de activación del protector de la aguja (señalados mediante asteriscos* en la Figura 1) para evitar que el protector de la aguja la cubra antes de tiempo.
- No use la jeringa precargada si cae sobre una superficie dura.
Compruebe la(s) jeringa(s) precargada(s) para asegurarse que
- El número de jeringas precargadas y la concentración son correctos
- Si su dosis es de 45 mg, tendrá una jeringa precargada de 45 mg de Otulfi.
- Si su dosis es de 90 mg, tendrá dos jeringas precargadas de 45 mg de Otulfi y tendrá que administrarse dos inyecciones. Elija dos lugares diferentes para estas inyecciones (e.j. uno en el muslo derecho y otro en el muslo izquierdo), e inyéctese una detrás de otra.
- Es el medicamento correcto.
- No ha expirado la fecha de caducidad.
- La jeringa precargada no está dañada.
- La solución en la jeringa precargada sea transparente y de incolora a ligeramente parda‑amarilla.
- La solución en la jeringa precargada no tenga un color anormal, esté turbia o contenga partículas extrañas.
- La solución en la jeringa precargada no esté congelada.
Prepare todo el material que necesita y colóquelo en una superficie limpia. Incluyendo toallitas antisépticas, algodón o gasa y un recipiente para objetos punzantes.
- Elija y prepare el lugar de inyección:
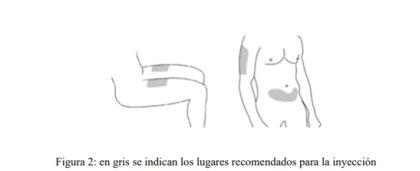
Elija el lugar de inyección (ver Figura 2).
- Otulfi se administra mediante inyección debajo de la piel (por vía subcutánea).
- Algunos lugares apropiados para la inyección son la parte superior del muslo o la zona de la tripa (el abdomen) como mínimo a 5 cm del ombligo.
- En la medida de lo posible, no use zonas de piel que muestren signos de psoriasis.
- Si otra persona le administra la inyección, entonces él o ella pueden elegir también la parte superior del brazo como lugar de inyección.
Prepare el lugar de inyección
- Lávese las manos muy bien con jabón y agua templada.
- Limpie la piel del lugar de inyección con una toallita antiséptica.
- No vuelva a tocar esta zona antes de ponerse la inyección.
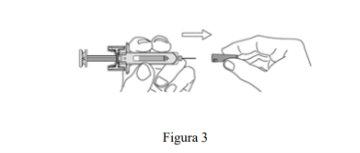
- Retire la tapa de la aguja (ver Figura 3):
- La tapa de la aguja nodebe retirarse hasta que no esté listo para inyectarse.
- Coja la jeringa precargada, y sujete el cuerpo de la jeringa precargada con una mano.
- Desprenda la tapa de la aguja y deshágase de ella. No toque el émbolo mientras hace esto
- Puede que observe una burbuja de aire en la jeringa precargada o una gota de líquido al final de la aguja. Ambas son normales y no es necesario eliminarlas.
- No toque la aguja ni permita que ésta toque ninguna superficie.
- No utilice la jeringa precargada si se ha caído sin la tapa de la aguja. Si esto sucede, comuníqueselo a su médico o farmacéutico.
- Inyecte la dosis inmediatamente después de retirar la tapa de la aguja.
- Inyecte la dosis:
- Sujete la jeringa precargada con una mano entre los dedos índice y corazón, coloque el pulgar sobre la cabeza del émbolo y con la otra mano pellizque con cuidado un pliego de piel desinfectada con los dedos pulgar e índice. No apriete.
- No retire el émbolo en ningún momento.
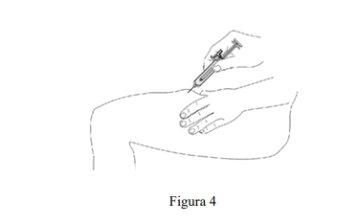
- Con un solo movimiento rápido, introduzca la aguja a través de la piel hasta donde pueda llegar (ver Figura 4).
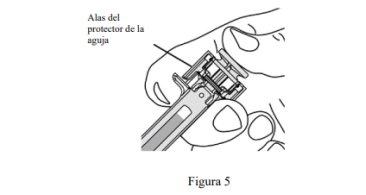
- Inyecte todo el medicamento empujando el émbolo hasta que la cabeza de éste se encuentre por completo entre las alas del protector de la aguja (ver Figura 5).
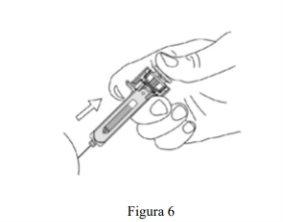
- Cuando haya empujado el émbolo hasta donde se lo permita, mantenga la presión sobre la cabeza del émbolo, saque la aguja y suelte la piel (ver Figura 6).
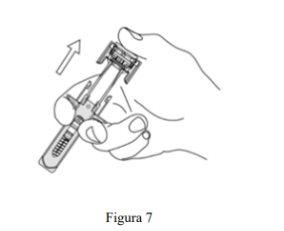
- Retire lentamente el pulgar de la cabeza del émbolo para que la jeringa vacía avance hasta que la aguja quede completamente cubierta por el protector de la aguja, como se muestra en la Figura 7:
- Después de la inyección:
- Presione el lugar de la inyección con una toallita antiséptica durante unos segundos después de la inyección.
- Puede aparecer una pequeña cantidad de sangre o líquido en el lugar de la inyección. Esto es normal.
- Puede presionar con un algodón o una gasa el lugar de la inyección y mantenerlo durante 10 segundos.
- No frote la piel en el lugar de inyección. Puede cubrir el lugar de la inyección con una tirita, si es necesario.
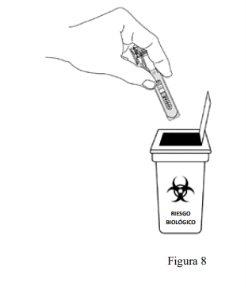
- Eliminación:
- Las jeringas utilizadas deben depositarse en un contenedor resistente a perforaciones, semejante a un contenedor para objetos punzo-cortantes (ver Figura 8). Por su seguridad y salud y por la seguridad de los demás, nunca vuelva a usar la jeringa. Elimine su contenedor para objetos punzo-cortantes de acuerdo a su normativa local.
- Las toallitas antisépticas y otros materiales pueden ser desechados en la basura.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a OTULFI 45 MG SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE PERFUSION, 130 mgPrincipio activo: UstekinumabFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE, 45 mgPrincipio activo: UstekinumabFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE, 90 mgPrincipio activo: UstekinumabFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para OTULFI 45 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de OTULFI 45 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






