OROXELAM 2,5 MG SOLUCION BUCAL

Cómo usar OROXELAM 2,5 MG SOLUCION BUCAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Oroxelam 2,5 mg solución bucal
Oroxelam 5 mg solución bucal
Oroxelam 7,5 mg solución bucal
Oroxelam 10 mg solución bucal
midazolam
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Oroxelam y para qué se utiliza
- Qué necesita saber antes de empezar a usar Oroxelam
- Cómo usar Oroxelam
- Posibles efectos adversos
- Conservación de Oroxelam
- Contenido del envase e información adicional
1. Qué es Oroxelam y para qué se utiliza
Oroxelam solución bucal contiene un medicamento llamado midazolam. Midazolam pertenece a un grupo de medicamentos conocidos como benzodiazepinas.
Oroxelam se utiliza para detener una crisis convulsiva súbita y prolongada en lactantes desde 3 meses a adultos.
En lactantes de 3 meses a menores de 6 meses, el tratamiento se debe administrar únicamente en un hospital en el que se pueda monitorizar al paciente y que cuente con equipo de reanimación.
Este medicamento debe ser utilizado únicamente por padres/cuidadores cuando se haya diagnosticado epilepsia al paciente.
2. Qué necesita saber antes de empezar a usar Oroxelam
- No use Oroxelam si el paciente tiene:
- alergia al midazolam, a las benzodiazepinas (como el diazepam) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- una enfermedad de los nervios y músculos que produce debilidad muscular (miastenia grave);
- serias dificultades respiratorias en descanso (Oroxelam puede hacer que las dificultades respiratorias empeoren);
- una enfermedad que produce interrupciones frecuentes de la respiración mientras se duerme (síndrome de apnea del sueño);
- problemas hepáticos graves.
Advertencias y precauciones:
Niños:
Consulte a su médico o farmacéutico antes de empezar a usar Oroxelam si el paciente:
- Tiene una afección renal, hepática o cardiaca;
- Tiene una afección pulmonar que produce dificultad respiratoria de forma periódica.
Adultos:
Consulte a su médico o farmacéutico antes de empezar a usar Oroxelam si:
- Usted es mayor de 60 años.
- Usted padece una enfermedad crónica (como insuficiencia respiratoria, o insuficiencia renal, hepática o cardíaca).
- Usted está debilitado (tiene una enfermedad que le hace sentir muy débil, agotado y sin energía).
Este medicamento puede hacer que las personas se olviden de lo ocurrido después de que se les haya administrado. Se debe observar detenidamente a los pacientes después de administrarles este medicamento.
Este medicamento se debe evitar en pacientes con antecedentes de alcoholismo o toxicomanía.
Es más probable que ocurran incidentes potencialmente mortales entre los pacientes con dificultades respiratorias o problemas cardiacos, especialmente cuando se administran dosis más altas de Oroxelam.
Niños menores de 3 meses: Oroxelam no se debe administrar a niños menores de 3 meses debido a la falta de información en este grupo de edad.
Pacientes de edad avanzada:
Los pacientes de edad avanzada son más sensibles a los efectos de las benzodiazepinas.
Si tiene alguna duda sobre si algo de lo anterior es aplicable al paciente, consulte a su
médico o farmacéutico antes de administrar este medicamento.
Otros medicamentos y Oroxelam
Informe a su médico o farmacéutico si el paciente está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento. Si tiene alguna duda sobre algún medicamento que el paciente está tomando y que pueda afectar al uso de Oroxelam, consulte a su médico o farmacéutico.
Esto es sumamente importante, ya que el uso de más de un medicamento al mismo tiempo puede potenciar o debilitar el efecto de los medicamentos tomados.
Los efectos de Oroxelam pueden intensificarse con los siguientes medicamentos:
- antiepilépticos (para tratar la epilepsia), p. ej.: fenitoína
- antibióticos, p. ej.: eritromicina, claritromicina
- antifúngicos, p. ej.: ketoconazol, voriconazol, fluconazol, itraconazol, pozaconazol
- medicamentos para las úlceras, p. ej.: cimetidina, ranitidina y omeprazol
- medicamentos utilizados para tratar la tensión arterial, p. ej.: diltiazem, verapamilo
- algunos medicamentos utilizados para el VIH y SIDA, p. ej.: saquinavir, combinación de lopinavir/ritonavir
- analgésicos narcóticos (calmantes muy fuertes), p. ej.: fentanilo
- medicamentos utilizados para reducir la grasa de la sangre, p. ej.: atorvastatina
- medicamentos utilizados para tratar las náuseas, p. ej.: nabilona
- hipnóticos (medicamentos para inducir el sueño)
- antidepresivos sedantes (medicamentos para tratar la depresión que producen sueño)
- sedantes (medicamentos para ayudar a relajarse)
- anestésicos (medicamentos para aliviar el dolor)
- antihistamínicos (medicamentos para tratar alergias)
Los efectos de Oroxelam pueden reducirse con los siguientes medicamentos:
- rifampicina (se utiliza para tratar la tuberculosis)
- xantinas (se utilizan para tratar el asma)
- la hierba de San Juan (un medicamento a base de plantas). Se debe evitar en los pacientes que tomen Oroxelam.
Oroxelam puede aumentar el efecto de algunos relajantes musculares, p. ej.: baclofeno (produciendo un aumento del sueño). Este medicamento también puede hacer que algunos medicamentos dejen de funcionar igual de bien, p. ej.: levodopa (un medicamento que se utiliza para tratar la enfermedad de Parkinson).
Consulte a su médico o farmacéutico para obtener más información sobre los medicamentos que el paciente debe evitar mientras toma Oroxelam.
Uso de Oroxelam con alimentos y bebidas
El paciente no debe beber alcohol mientras toma Oroxelam. El alcohol puede incrementar los efectos sedantes de este medicamento y producirle mucho sueño.
El paciente no debe beber zumo de pomelo mientras toma Oroxelam. El zumo de pomelo puede incrementar los efectos sedantes de este medicamento y producirle mucho sueño.
Embarazo
Si la paciente que va a recibir este medicamento está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
La administración de dosis altas de Oroxelam durante los últimos 3 meses de embarazo puede producir latido cardiaco anómalo en el feto. Los niños nacidos después de la administración de este medicamento durante el parto pueden también presentar dificultad para mamar, dificultades respiratorias y un tono muscular pobre al nacer.
Lactancia
Informe al médico si la paciente está dando el pecho. A pesar de que pequeñas cantidades de Oroxelam pueden pasar a la leche materna, puede que no sea necesario suspender la lactancia materna. El médico le aconsejará sobre si la paciente debe amamantar al bebé después de recibir una sola dosis de este medicamento.
Conducción y uso de máquinas
Oroxelam puede hacer que el paciente se sienta somnoliento, se olvide de las cosas o vea afectada su concentración y coordinación. Esto puede interferir en la ejecución de tareas que requieren habilidad tales como conducir, montar en bicicleta o utilizar máquinas.
Tras recibir este medicamento, el paciente no debe conducir, montar en bicicleta ni utilizar máquinas hasta que se haya recuperado por completo. Pregunte a su médico si necesita más información.
Oroxelam contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por jeringa oral , esto es, esencialmente “exento de sodio”.
3. Cómo administrar Oroxelam
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Dosis
El médico le recetará la dosis de Oroxelam apropiada, que normalmente va a depender de la edad del paciente. Cada una de las dosis tiene un color diferente, que se muestra en la caja, el tubo y la jeringa que contiene el medicamento.
Dependiendo de la edad, el paciente recibirá una de las siguientes dosis, en un envase etiquetado expresamente por colores:
Rango de edad | Dosis | Color de la Etiqueta |
3 meses a menores de 1 año | 2,5 mg | Amarilla |
1 año a menores de 5 años | 5 mg | Azul |
5 años a menores de 10 años | 7,5 mg | Morada |
10 años a adultos | 10 mg | Naranja |
Una jeringa oral contiene una dosis completa. No administre más de una dosis.
Los lactantes de 3 meses a menores de 6 meses únicamente deben recibir tratamiento en un hospital en el que se pueda monitorizar al paciente y que cuente con equipo de reanimación.
Preparación para la administración de este medicamento
Si el paciente presenta una crisis convulsiva, deje que su cuerpo se mueva libremente, no intente sujetarle. Muévale solamente si corre peligro por su proximidad a, por ejemplo, aguas profundas, fuego u objetos cortantes.
Apoye la cabeza de del paciente sobre algún objeto acolchado como, por ejemplo, un cojín o en su regazo. Compruebe que el medicamento contiene la dosis correcta para el paciente, según para su edad.
Cómo administrar este medicamento
Pida a un médico, farmacéutico o enfermero que le enseñen cómo tomar o administrar este medicamento. En caso de duda, pregunte siempre a su médico, farmacéutico o enfermero.
La información sobre cómo administrar este medicamento también aparece en la etiqueta del tubo.
Oroxelam no se debe inyectar. No se debe colocar ninguna aguja en la jeringa.
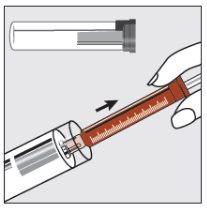
Paso 1 | |
| Sujetar el tubo de plástico, romper el precinto por un extremo y retirar la cápsula de cierre. Sacar la jeringa del tubo. |
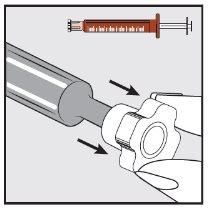
Paso 2 | |
| Retirar la cápsula de cierre transparente de la punta de la jeringa y desecharla de forma segura. |
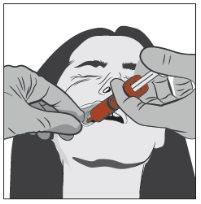
Paso 3 | |
| Con ayuda del dedo índice y el pulgar, pellizcar y tirar suavemente hacia atrás de la mejilla del paciente. Colocar la punta de la jeringa en la parte posterior del espacio entre el interior de la mejilla y la encía inferior. |
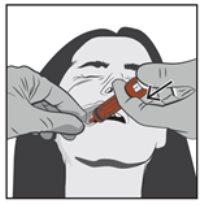
Paso 4 | |
| Presionar lentamente el émbolo de la jeringa hasta que se detenga. Se debe introducir lentamente toda la solución en el espacio entre la encía y la mejilla (cavidad bucal). Si lo prescribe su médico (para volúmenes grandes y/o pacientes más pequeños), se puede administrar lentamente aproximadamente la mitad de la dosis en un lado de la boca y, a continuación, la otra mitad en el otro lado de la boca del paciente. |
Cuándo llamar a una ambulancia
Siga SIEMPRE las recomendaciones de tratamiento proporcionadas por el médico del paciente o tal como le indicó el profesional sanitario. En caso de duda, solicite ayuda médica urgente si:
- La crisis convulsiva no remite en un plazo de 10 minutos.
- Es incapaz de vaciar el contenido de la jeringa o derrama algo del mismo.
- La respiración del paciente se ralentiza o detiene (p. ej.: respiración lenta o superficial o labios azules).
- Observa signos de infarto de miocardio que pueden incluir dolor torácico o dolor que irradia al cuello y hombros y se extiende hasta el brazo izquierdo.
- El paciente vomita y la crisis convulsiva no remite en un plazo de 10 minutos.
- Le administra demasiado Oroxelam y observa signos de sobredosis que incluyen:
- Somnolencia, cansancio, fatiga
- Confusión o desorientación
- Ausencia de reflejo en la rodilla o de respuesta a un pellizco
- Dificultades respiratorias (respiración lenta o superficial)
- Tensión arterial baja (vértigo y sensación de desmayo)
- Coma
Conserve la jeringa para mostrársela al personal sanitario de la ambulancia o al médico.
No administre más cantidad de medicamento de la prescrita por el médico para el paciente.
Si la paciente vomita
- No administre al paciente otra dosis de Oroxelam.
- Si la crisis convulsiva no remite en el plazo de 10 minutos, llame a una ambulancia.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Solicite atención médica inmediata o llame por teléfono para pedir una ambulancia si el paciente experimenta los siguientes efectos adversos:
- Dificultad respiratoria grave, p. ej.: respiración lenta o superficial o labios azules. En muy raros casos, podrá pararse la respiración.
- Infarto de miocardio. Los signos pueden incluir dolor torácico que puede irradiarse al cuello y hombros del paciente y extenderse hasta su brazo izquierdo.
- Hinchazón de cara, labios, lengua o garganta que dificulte tragar o respirar, o piel pálida, pulso débil y rápido, o sensación de pérdida del conocimiento. Es posible que esté teniendo una reacción alérgica grave.
Otros efectos adversos
Si el paciente experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto.
Efectos adversos frecuentes (pueden afectar hasta a 1 de cada 10 personas):
- Sentirse y estar enfermo (nauseas y vómitos).
- Somnolencia o estar menos consciente
Efectos adversos poco frecuentes (pueden afectar hasta a 1 de cada 100 personas):
- Erupción cutánea, urticaria (ronchas), picor
Efectos adversos muy raros (pueden afectar hasta a 1 de cada 10.000 personas):
- Agitación, inquietud, hostilidad, ira o agresión, excitación, confusión, euforia (sensación excesiva de alegría o excitación) o alucinaciones (ver y posiblemente oír cosas que realmente no ocurren)
- Espasmos musculares y temblores musculares (temblor de los músculos que no se puede controlar)
- Nivel de alerta reducido
- Dolor de cabeza
- Mareos
- Dificultad para coordinar los músculos
- Crisis convulsivas (convulsiones)
- Pérdida transitoria de la memoria. La duración depende de la cantidad de Oroxelam administrada.
- Tensión arterial baja, frecuencia cardiaca lenta o enrojecimiento de la cara y cuello (rubefacción)
- Espasmo laríngeo (contracción de las cuerdas vocales que produce dificultad respiratoria y ruido al respirar)
- Estreñimiento
- Sequedad de boca
- Cansancio
- Hipo
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Oroxelam
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en las etiquetas del tubo y de la jeringa para uso oral, después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar por debajo de 30 ºC
Mantener la jeringa para uso oral en el tubo de plástico protector. No utilice este medicamento si el envase está abierto o dañado.
E
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de oroxelam
El principio activo es midazolam
- Oroxelam 2,5 mg - Cada jeringa precargada para uso oral contiene midazolam hidrocloruro equivalente a 2,5 mg de midazolam en 0,5 ml de solución.
- Oroxelam 5 mg - Cada jeringa precargada para uso oral contiene midazolam hidrocloruro equivalente a 5 mg de midazolam en 1 ml de solución.
- Oroxelam 7,5 mg - Cada jeringa precargada para uso oral contiene midazolam hidrocloruro equivalente a 7,5 mg de midazolam en 1,5 ml de solución.
- Oroxelam 10 mg - Cada jeringa precargada para uso oral contiene midazolam hidrocloruro equivalente a 10 mg de midazolam en 2 ml de solución.
Los demás componentes son cloruro de sodio, agua purificada, ácido clorhídrico e hidróxido de sodio (para ajustar el pH).
Aspecto del producto y contenido del envase
Oroxelam 2,5 mg – envase con etiqueta amarilla
Oroxelam 5 mg – envase con etiqueta azul
Oroxelam 7,5 mg – envase con etiqueta morada
Oroxelam 10 mg – envase con etiqueta naranja
Oroxelam solución bucal es un líquido transparente e incoloro.
Se presenta en una jeringa precargada para uso oral de color ámbar, sin aguja, con émbolo y tapón. Cada jeringa para uso oral viene envasada individualmente en un tubo de plástico protector.
Oroxelam se presenta en cajas que contienen 2 ó 4 jeringas precargadas para uso oral (de la misma dosis).
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Exeltis Healthcare, S.L.
Avenida de Miralcampo, 7.
Polígono Industrial Miralcampo.
19200 Azuqueca de Henares.
Guadalajara.
España
Responsable de la fabricación
Laboratorios Liconsa S.A.
Av. de Miralcampo, 7
19200 Azuqueca de Henares
Guadalajara.
España
Este medicamento está autorizado en los estados miembro del espacio económico europeo con los siguientes nombres:
SE: Midazolam medical valley 2.5 mg, 5 mg, 7.5 mg, 10 mg munhålelösning.
FI: Midazolam medical valley 2.5 mg, 5 mg, 7.5 mg, 10 mg liuos suuonteloon.
DE: Midazaxiro 2.5 mg, 5 mg, 7.5 mg, 10 mg lösung zur anwendung in der mundhöhle.
NO: Midazolam medical valley 2.5 mg, 5 mg, 7.5 mg, 10 mg munnvann, oppløsning.
NL: Midazolam xiromed 2.5 mg, 5 mg, 7.5 mg, 10 mg oplossing voor oromucosaal gebruik.
DK: Midazolam medical valley 2.5 mg, 5 mg, 7.5 mg, 10 mg mundhulevæske, opløsning.
IS: Midazolam medical valley 2.5 mg, 5 mg, 7.5 mg, 10 mg munnholslausn.
FR: Midazolam liconsa 2.5 mg, 5 mg, 7.5 mg, 10 mg solution buccale.
IE: Midazolam liconsa 2.5 mg, 5 mg, 7.5 mg, 10 mg oromucosal solution.
RO: Midazolam desitin 2.5 mg, 5 mg, 7.5 mg, 10 mg solu?ie bucofaringiana.
ES: Oroxelam 2,5 mg, 5 mg, 7,5 mg, 10 mg solución bucal
PL: Soloxelam.
IT: Oroxelam.
Fecha de la última revisión de este prospecto:marzo 2025
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a OROXELAM 2,5 MG SOLUCION BUCALForma farmacéutica: GEL/PASTA/LIQUIDO BUCAL, 10 mg midazolam hidrocloruroPrincipio activo: MidazolamFabricante: Neuraxpharm Pharmaceuticals S.L.Requiere recetaForma farmacéutica: GEL/PASTA/LIQUIDO BUCAL, 2,5 mg midazolam hidrocloruroPrincipio activo: MidazolamFabricante: Neuraxpharm Pharmaceuticals S.L.Requiere recetaForma farmacéutica: GEL/PASTA/LIQUIDO BUCAL, 5 mg midazolam hidrocloruroPrincipio activo: MidazolamFabricante: Neuraxpharm Pharmaceuticals S.L.Requiere receta
Médicos online para OROXELAM 2,5 MG SOLUCION BUCAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de OROXELAM 2,5 MG SOLUCION BUCAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes