
ORNIBEL 0,120 MG/0,015 MG CADA 24 HORAS SISTEMA DE LIBERACION VAGINAL EFG


Cómo usar ORNIBEL 0,120 MG/0,015 MG CADA 24 HORAS SISTEMA DE LIBERACION VAGINAL EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Ornibel 0,120 mg/0,015 mg cada 24 horas, sistema de liberación vaginal EFG
(Etonogestrel/Etinilestradiol)
Cosas importantes que debe saber acerca de los anticonceptivos hormonales combinados (AHCs):
- Son uno de los métodos anticonceptivos reversibles más fiables si se utilizan correctamente.
- Aumentan ligeramente el riesgo de sufrir un coágulo de sangre en las venas y arterias, especialmente en el primer año o cuando se reinicia el uso de un anticonceptivo hormonal combinado tras una pausa de 4 semanas o más.
- Esté alerta y consulte a su médico si cree que puede tener síntomas de un coágulo de sangre (ver sección 2 “Coágulos de sangre”).
Lea todo el prospecto detenidamente antes de empezar a usar Ornibel, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ornibel y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ornibel
2.1 Cuando no debe usar Ornibel
2.2 Advertencias y precauciones
Coágulos de sangre
Cáncer
2.3 Niños y adolescentes
2.4 Otros medicamentos y Ornibel
Pruebas de laboratorio
2.5 Embarazo y lactancia
2.6 Conducción y uso de máquinas
- Como usar Ornibel
3.1 Forma de ponerse y quitarse el Ornibel
3.2 Tres semanas dentro, una semana fuera
3.3 Cuando empezar con el primer anillo
3.4 Que hacer si…
Su anillo se expulsa accidentalmente de la vagina
Su anillo ha estado fuera de la vagina temporalmente
El anillo se abre
Se ha puesto más de un anillo
Si olvida ponerse un nuevo anillo después de la pausa sin anillo
Si ha olvidado quitarse el anillo
Si no aparece el periodo o regla
Si tiene un sangrado inesperado
Si desea cambiar el día en que empieza su periodo o regla
Si desea retrasar su periodo o regla
3.5 Si desea dejar de usar Ornibel.
- Posibles efectos adversos
- Conservación de Ornibel
- Contenido del envase e información adicional
Composición de Ornibel
Aspecto de Ornibel y contenido del envase
Titular de la Autorización de Comercialización y Responsable de la fabricación
1. Qué es Ornibel y para qué se utiliza
Ornibel es un anillo vaginal anticonceptivo que se utiliza para evitar el embarazo. Cada anillo contiene una pequeña cantidad de dos hormonas sexuales femeninas, etonogestrel y etinilestradiol. El anillo libera lentamente estas hormonas al torrente sanguíneo. Dada la pequeña cantidad de hormonas liberadas, Ornibel es un anticonceptivo hormonal de baja dosis.
Como Ornibel libera dos tipos diferentes de hormonas, es un anticonceptivo hormonal combinado
Ornibel actúa como la píldora anticonceptiva combinada (la “Píldora”), pero en vez de tomar una píldora cada día, el anillo se utiliza durante 3 semanas seguidas. Ornibel libera dos hormonas sexuales femeninas que impiden que los ovarios liberen un óvulo. Si no se libera ningún óvulo, no puede quedarse embarazada
2. Qué necesita saber antes de empezar a usar Ornibel
Consideraciones generales
Antes de empezar a usar Ornibel debe leer la información acerca de los coágulos de sangre en la sección 2. Es particularmente importante que lea los síntomas de un coágulo de sangre (ver sección 2 “Coágulos de sangre”).
En este prospecto se describen diversas situaciones en las que debe usted dejar de usar Ornibel, o en las que Ornibel puede ser menos fiable. En estas situaciones, no debe mantener relaciones sexuales, o debe tomar medidas anticonceptivas adicionales no hormonales, como el preservativo u otro método de barrera. Noutilice el método del ritmo o el de la temperatura. Puede ser que estos métodos no sean fiables ya que Ornibel altera los cambios mensuales de temperatura del cuerpo y el moco cervical.
Ornibel, al igual que otros anticonceptivos hormonales, no protege contra la infección por VIH (SIDA) ni cualquier otra enfermedad de transmisión sexual.
2.1 Cuando no debe usar Ornibel
No debe usar Ornibel si tiene alguna de las afecciones enumeradas a continuación. Informe a su médico si tiene alguna de las afecciones enumeradas a continuación. Su médico comentará con usted qué otra forma de anticoncepción sería más adecuada.
- Si tiene (o ha tenido alguna vez) un coágulo de sangre en un vaso sanguíneo de las piernas (trombosis venosa profunda, TVP), en los pulmones (embolia pulmonar, EP) o en otros órganos.
- Si sabe que padece un trastorno que afecta a la coagulación de la sangre: por ejemplo, deficiencia de proteína C, deficiencia de proteína S, deficiencia de antitrombina III, factor V Leiden o anticuerpos antifosfolípidos.
- Si necesita una operación o si pasa mucho tiempo sin ponerse de pie (ver sección “Coágulos de sangre”).
- Si ha sufrido alguna vez un ataque al corazón o un ictus.
- Si tiene (o ha tenido alguna vez) una angina de pecho (una afección que provoca fuerte dolor en el pecho y puede ser el primer signo de un ataque al corazón) o un accidente isquémico transitorio (AIT, síntomas temporales de ictus)).
- Si tiene alguna de las siguientes enfermedades que pueden aumentar su riesgo de formación de un coágulo en las arterias:
- Diabetes grave con lesión de los vasos sanguíneos.
- Tensión arterial muy alta.
- Niveles muy altos de grasa en la sangre (colesterol o triglicéridos).
- Una afección llamada hiperhomocisteinemia.
- Si tiene (o ha tenido alguna vez) un tipo de migraña llamada “migraña con aura”.
- Si tiene o ha tenido inflamación del páncreas (pancreatitis), asociada a niveles altos de grasas en su sangre.
- Si tiene o ha tenido una enfermedad grave del hígado y su hígado aún no funciona de forma normal.
- Si tiene o ha tenido un tumor benigno o maligno en el hígado.
- Si tiene, ha tenido o podría tener un cáncer de mama o de los órganos genitales.
- Si tiene algún sangrado vaginal de origen desconocido.
- Si es alérgica a etinilestradiol o etonogestrel, o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Si cualquiera de estas circunstancias se presenta por primera vez mientras utiliza Ornibel, quítese el anillo inmediatamente y consulte a su médico. Mientras tanto, utilice medidas anticonceptivas no hormonales.
No utilice Ornibel si tiene hepatitis C o está tomando medicamentos que contienen ombitasvir / paritaprevir / ritonavir y dasabuvir o glecaprevir / pibrentasvir o sofosbuvir/velpatasvir/voxilaprevir (ver también la sección 2.4 “Uso de Ornibel con otros medicamentos”)
2.2 Advertencias y precauciones
Cuándo debe consultar a su médico?
Busque asistencia médica urgente
- Si nota posibles signos de un coágulo de sangre que pueden significar que está sufriendo un coágulo de sangre en la pierna (es decir, trombosis venosa profunda), un coágulo de sangre en el pulmón (es decir, embolia pulmonar), un ataque al corazón o un ictus (ver sección “Coágulos de sangre” a continuación).
Para obtener una descripción de los síntomas de estos efectos adversos graves, consulte “Cómo reconocer un coágulo de sangre”
Informe a su médico si sufre cualquiera de las siguientes afecciones.
Si la afección se desarrolla o empeora mientras está usando Ornibel, también debe informar a su médico.
- Si algún familiar directo padece o ha padecido alguna vez cáncer de mama.
- Si sufre epilepsia (ver la sección 2.4. “Otros medicamentos y Ornibel”).
- Si tiene una enfermedad del hígado (por ejemplo, ictericia) o de la vesícula (por ejemplo, piedras en la vesícula).
- Si tiene enfermedad de Crohn o colitis ulcerosa (enfermedad intestinal inflamatoria crónica).
- Si tiene lupus eritematoso sistémico (LES, una enfermedad que afecta su sistema natural de defensa).
- Si tiene síndrome urémico hemolítico (SUH, un trastorno de la coagulación de la sangre que provoca insuficiencia en el riñón).
- Si tiene anemia de células falciformes (una enfermedad hereditaria de los glóbulos rojos).
- Si tiene niveles elevados de grasa en la sangre (hipertrigliceridemia) o antecedentes familiares conocidos de esta afección. La hipertrigliceridemia se ha asociado a un mayor riesgo de padecer pancreatitis (inflamación del páncreas).
- Si necesita una operación o pasa mucho tiempo sin ponerse de pie (ver sección 2 “Coágulos de sangre”).
- Si acaba de dar a luz corre mayor riesgo de sufrir coágulos de sangre. Debe preguntar a su médico cuándo puede empezar a tomar Ornibel tras el parto.
- Si tiene una inflamación de las venas que hay debajo de la piel (tromboflebitis superficial).
- Si tiene varices.
- Si padece alguna enfermedad que apareció por primera vez o empeoró durante un embarazo o uso anterior de hormonas sexuales (por ejemplo, pérdida de oído, porfiria [una enfermedad de la sangre], herpes del embarazo [erupción cutánea con vesículas durante el embarazo], o corea de Sydenham [enfermedad nerviosa en la que se producen movimientos involuntarios] .
- Si tiene o ha tenido alguna vez cloasma (manchas de color amarillento-marrón en la piel, llamadas “manchas del embarazo”, particularmente en la cara); si este es el caso, evite la exposición excesiva al sol o los rayos ultravioleta.
- Si tiene trastornos que hagan difícil el uso de Ornibel, por ejemplo si padece estreñimiento, prolapso de la cérvix uterina o siente dolor durante las relaciones sexuales.
- Si tiene una micción urgente, frecuente, con ardor y/o dolorosa, y no puede localizar el anillo en la vagina. Estos síntomas pueden indicar que Ornibel se ha colocado accidentalmente en la vejiga urinaria.
- Si experimenta síntomas de angioedema como hinchazón de cara, lengua y/o garganta y/o dificultad para tragar o urticaria con posible dificultad para respirar, contacte con un médico de forma inmediata. Los productos que contienen estrógenos pueden causar o empeorar los síntomas de angioedema hereditario y adquirido.
COÁGULOS DE SANGRE
El uso de un anticonceptivo hormonal combinado como Ornibel aumenta su riesgo de sufrir un coágulo de sangre en comparación con no usarlo. En raras ocasiones un coágulo de sangre puede bloquear vasos sanguíneos y provocar problemas graves.
Se pueden formar coágulos de sangre:
- En las venas (lo que se llama “trombosis venosa”, “tromboembolismo venoso” o TEV).
- En las arterias (lo que se llama “trombosis arterial”, “tromboembolismo arterial” o TEA).
La recuperación de los coágulos de sangre no es siempre completa. En raras ocasiones puede haber efectos graves duraderos o, muy raramente, pueden ser mortales.
Es importante recordar que el riesgo global de un coágulo de sangre perjudicial debido a Ornibel es pequeño.
CÓMO RECONOCER UN COÁGULO DE SANGRE
Busque asistencia médica urgente si nota alguno de los siguientes signos o síntomas.
¿Experimenta alguno de estos signos? | ¿Qué es posible que esté sufriendo? |
| Trombosis venosa profunda |
Si no está segura, consulte a un médico, ya que algunos de estos síntomas como la tos o la falta de aliento se pueden confundir con una afección más leve como una infección respiratoria (p. ej. un “catarro común”). | Embolia pulmonar |
Síntomas que se producen con más frecuencia en un ojo:
| Trombosis de las venas retinianas (coágulo de sangre en el ojo). |
| Ataque al corazón. |
A veces los síntomas de un ictus pueden ser breves, con una recuperación casi inmediata y completa, pero de todos modos debe buscar asistencia médica urgente ya que puede correr riesgo de sufrir otro ictus. | Ictus |
| Coágulos de sangre que bloquean otros vasos sanguíneos. |
COÁGULOS DE SANGRE EN UNA VENA
¿Qué puede ocurrir si se forma un coágulo de sangre en una vena?
- El uso de anticonceptivos hormonales combinados se ha relacionado con un aumento del riesgo de coágulos de sangre en las venas (trombosis venosa). No obstante, estos efectos adversos son raros. Se producen con más frecuencia en el primer año de uso de un anticonceptivo hormonal combinado.
- Si se forma un coágulo de sangre en una vena de la pierna o del pie, puede provocar trombosis venosa profunda (TVP).
- Si un coágulo de sangre se desplaza desde la pierna y se aloja en el pulmón puede provocar una embolia pulmonar.
- En muy raras ocasiones se puede formar un coágulo en una vena de otro órgano como el ojo (trombosis de las venas retinianas).
¿Cuándo es mayor el riesgo de presentar un coágulo de sangre en una vena?
El riesgo de presentar un coágulo de sangre en una vena es mayor durante el primer año en el que se toma un anticonceptivo hormonal combinado por primera vez. El riesgo puede ser mayor también si vuelve a empezar a tomar un anticonceptivo hormonal combinado (el mismo medicamento o un medicamento diferente) después de una interrupción de 4 semanas o más.
Después del primer año, el riesgo disminuye, pero siempre es algo mayor que si no estuviera tomando un anticonceptivo hormonal combinado.
Cuando deja de utilizar Ornibel, su riesgo de presentar un coágulo de sangre regresa a la normalidad en pocas semanas.
¿Cuál es el riesgo de presentar un coágulo de sangre?
El riesgo depende de su riesgo natural de TEV y del tipo de anticonceptivo hormonal combinado que esté tomando.
El riesgo global de presentar un coágulo de sangre en la pierna o en el pulmón (TVP o EP) con Ornibel es pequeño.
- De cada 10.000 mujeres que no usan un anticonceptivo hormonal combinado y que no están embarazadas, unas 2 presentarán un coágulo de sangre en un año.
- De cada 10.000 mujeres que usan un anticonceptivo hormonal combinado que contiene levonorgestrel, noretisterona o norgestimato, unas 5-7 presentarán un coágulo de sangre en un año.
- De cada 10.000 mujeres que usan un anticonceptivo hormonal combinado que contiene norelgestromina, o etonogestrel como Ornibel, entre unas 6 y 12 mujeres presentarán un coágulo de sangre en un año.
- El riesgo de presentar un coágulo de sangre dependerá de sus antecedentes personales (ver “Factores que aumentan su riesgo de un coágulo de sangre” más adelante).
Riesgo de presentar un coágulo de sangre en un año | |
Mujeres que no utilizanun comprimido/parche/anillo hormonal combinado y que no están embarazadas | Unas 2 de cada 10.000 mujeres |
Mujeres que utilizan un comprimido anticonceptivo hormonal combinado que contiene levonorgestrel, noretisterona o norgestimato | Unas 5-7 de cada 10.000 mujeres |
Mujeres que utilizan Ornibel | Unas 6-12 de cada 10.000 mujeres |
Factores que aumentan su riesgo de un coágulo de sangre en una vena
El riesgo de tener un coágulo de sangre con Ornibel es pequeño, pero algunas afecciones aumentan el riesgo. Su riego es mayor:
- Si tiene exceso de peso (índice de masa corporal o IMC superior a 30 kg/m2).
- Si alguno de sus parientes próximos ha tenido un coágulo de sangre en la pierna, pulmón u otro órgano a una edad temprana (es decir, antes de los 50 años aproximadamente). En este caso podría tener un trastorno hereditario de la coagulación de la sangre.
- Si necesita operarse o si pasa mucho tiempo sin ponerse de pie debido a una lesión o enfermedad o si tiene la pierna escayolada. Tal vez haya que interrumpir el uso de Ornibel varias semanas antes de la intervención quirúrgica o mientras tenga menos movilidad. Si necesita interrumpir el uso de Ornibel pregúntele a su médico cuándo puede empezar a usarlo de nuevo.
- Al aumentar la edad (en especial por encima de unos 35 años).
- Si ha dado a luz hace menos de unas semanas.
El riesgo de presentar un coágulo de sangre aumenta cuantas más afecciones tenga.
Los viajes en avión (más de 4 horas) pueden aumentar temporalmente el riesgo de un coágulo de sangre, en especial si tiene alguno de los demás factores de riesgo enumerados.
Es importante informar a su médico si sufre cualquiera de las afecciones anteriores, aunque no esté segura. Su médico puede decidir que hay que interrumpir el uso de Ornibel.
Si alguna de las afecciones anteriores cambia mientras está utilizando Ornibel, por ejemplo un pariente próximo experimenta una trombosis sin causa conocida o usted aumenta mucho de peso, informe a su médico.
COÁGULOS DE SANGRE EN UNA ARTERIA
¿Qué puede ocurrir si se forma un coágulo de sangre en una arteria?
Al igual que un coágulo de sangre en una vena, un coágulo en una arteria puede provocar problemas graves. Por ejemplo, puede provocar un ataque al corazón o un ictus.
Factores que aumentan su riesgo de un coágulo de sangre en una arteria
Es importante señalar que el riesgo de un ataque al corazón o un ictus por utilizar Ornibel es muy pequeño, pero puede aumentar:
- Con la edad (por encima de unos 35 años).
- Si fuma.Cuando utiliza un anticonceptivo hormonal combinado como Ornibel se le aconseja que deje de fumar. Si no es capaz de dejar de fumar y tiene más de 35 años, su médico puede aconsejarle que utilice un tipo de anticonceptivo diferente.
- Si tiene sobrepeso.
- Si tiene la tensión alta.
- Si algún pariente próximo ha sufrido un ataque al corazón o un ictus a una edad temprana (menos de unos 50 años). En este caso usted también podría tener mayor riesgo de sufrir un ataque al corazón o un ictus.
- Si usted o alguno de sus parientes próximos tiene un nivel elevado de grasa en la sangre (colesterol o triglicéridos).
- Si padece migrañas, especialmente migrañas con aura.
- Si tiene un problema de corazón (trastorno de las válvulas, alteración del ritmo cardíaco llamado fibrilación auricular).
- Si tiene diabetes.
Si tiene una o más de estas afecciones o si alguna de ellas es especialmente grave, el riesgo de presentar un coágulo de sangre puede verse incrementado aún más.
Si alguna de las afecciones anteriores cambia mientras está utilizando Ornibel, por ejemplo empieza a fumar, un pariente próximo experimenta una trombosis sin causa conocida o usted aumenta mucho de peso, informe a su médico.
Cáncer
La siguiente información se ha obtenido de estudios con anticonceptivos orales combinados y podría ser aplicable a Ornibel. No se dispone de información sobre la administración por vía vaginal de hormonas anticonceptivas (como Ornibel).
Se han observado casos de tumores de mama con una frecuencia ligeramente mayor en mujeres que utilizan píldoras anticonceptivas, pero se desconoce si esto se debe al tratamiento. Por ejemplo, podría ser que se encontraran más tumores en las mujeres que usan píldoras anticonceptivas porque acuden a revisión médica con más frecuencia. Este aumento de frecuencia disminuye gradualmente después de interrumpir el tratamiento.
Es importante examinar sus pechos regularmente e informar a su médico si nota algún bulto. Informe a su médico si algún pariente cercano tiene o ha tenido un cáncer de mama (vea la sección 2.2 “Advertencias y precauciones”).
Rara vez se han comunicado casos de tumores de hígado benignos, y en menos casos todavía tumores de hígado malignos, en mujeres que usan la píldora anticonceptiva. Contacte con su médico si tiene un dolor fuerte y poco común en el abdomen.
En usuarias de la píldora combinada se ha observado que se produce con menos frecuencia cáncer de endometrio (el tejido que recubre el útero) o de ovarios. Esto también podría ser el caso de Ornibel, pero no ha sido confirmado.
Trastornos psiquiátricos:
Algunas mujeres que utilizan anticonceptivos hormonales como Ornibel han notificado depresión o un estado de ánimo deprimido. La depresión puede ser grave y a veces puede inducir pensamientos suicidas. Si experimenta alteraciones del estado de ánimo y síntomas depresivos, póngase en contacto con su médico para obtener asesoramiento médico adicional lo antes posible.
2.3 Niños y adolescentes
No se ha establecido la seguridad y eficacia de Ornibel en adolescentes menores de 18 años.
2.4 Otros medicamentos y Ornibel
Informe siempre a su médico sobre qué medicamentos o plantas medicinales está tomando. Informe también a cualquier médico o dentista (o farmacéutico) que le recete otro medicamento que está utilizando Ornibel. Ellos le podrán informar si necesita tomar alguna medida anticonceptiva complementaria (por ejemplo, el uso de preservativos masculinos) y si fuera necesario, durante cuánto tiempo, o bien si debe modificar el uso del otro medicamento.
Algunos medicamentos
- pueden tener una influencia en los niveles de Ornibel en sangre
- pueden hacer que sea menos efectivo en la prevención del embarazo
- pueden causar sangrados inesperados.
Estos incluyen los medicamentos utilizados para el tratamiento de:
- la epilepsia (por ejemplo primidona, fenitoína, barbitúricos, carbamazepina, oxcarbazepina, topiramato, felbamato);
- tuberculosis (por ejemplo rifampicina);
- infección por VIH (por ejemplo ritonavir, nelfinavir, nevirapina, efavirenz);
- infección por el virus de la Hepatitis C (por ejemplo boceprevir, telaprevir);
- otras enfermedades infecciosas (por ejemplo griseofulvina);
- la presión alta en los vasos sanguíneos de los pulmones (bosentan);
- estados de ánimo depresivos (la planta medicinal Hierba de San Juan.
Si está tomando medicamentos o plantas medicinales que podrían hacer menos eficaz a Ornibel, debe utilizar también un método anticonceptivo de barrera (p.ej. preservativo masculino). Dado que el efecto de otro medicamento sobre Ornibel puede durar hasta 28 días después de suspender el medicamento, durante ese tiempo es necesario utilizar un método anticonceptivo de barrera adicional. Nota: No use Ornibel junto con un diafragma, capuchón cervical o preservativo femenino.
Ornibel puede influir sobre el efecto de otros medicamentos, por ejemplo:
- medicamentos que contienen ciclosporina
- el antiepiléptico lamotrigina (esto podría conllevar un aumento de la frecuencia de las convulsiones)
No use Ornibel si usted padece Hepatitis C y está tomando medicamentos que contienen omvitasvir/paritaprevir/ritonavir y dasabuvir o glecaprevir/pibrentasvir o sofosbuvir/velpatasvir/voxilaprevir, ya que estos medicamentos pueden causar aumentos en los parámetros que miden la función hepáticaen sangre (aumento de la enzima hepática ALT).
Su médico le prescribirá otro tipo de anticonceptivo antes de comenzar el tratamiento con estos medicamentos.
Ornibel se puede reiniciar aproximadamente 2 semanas después de completar este tratamiento. Consulte la sección 2.1 “Cuando no debe usar Ornibel”.
Consulte a su médico o farmacéutico antes de tomar cualquier medicamento.
Se pueden usar tampones mientras utiliza Ornibel. Póngase primero Ornibel y luego el tampón. Tenga especial cuidado al quitarse el tampón para que el anillo no sea expulsado accidentalmente. En caso de que se expulse, simplemente lave el anillo con agua fría o tibia y vuelva a ponérselo inmediatamente.
El anillo se puede abrir cuando se está utilizando también un producto vaginal como un lubricante o un tratamiento para la infección (consulte sección 3.4 “Qué hacer si....el anillo se abre”)
El uso de espermicidas o productos para los hongos vaginales no reduce la eficacia anticonceptiva de Ornibel.
Pruebas de laboratorio
Si le hacen análisis de sangre u orina, informe a su médico que usa Ornibel, ya que puede afectar al resultado de algunos análisis.
2.5 Embarazo y lactancia
No deben utilizar Ornibel mujeres embarazadas o que sospechen que pueden estarlo. Si se queda embarazada mientras usa Ornibel deberá quitarse el anillo y consultar a su médico.
Si desea dejar Ornibel porque quiere quedarse embarazada, vea la sección 3.5 “Si desea dejar de usar Ornibel”.
En general no se recomienda utilizar Ornibel en caso de que esté dando el pecho. Si desea usar Ornibel dando el pecho consulte a su médico.
2.6 Conducción y uso de máquinas
No es probable que Ornibel afecte su capacidad de conducir o utilizar máquinas
3. Cómo usar Ornibel
Puede colocarse y quitarse Ornibel usted misma. Su médico le indicará en qué momento empezar a utilizarlo por primera vez. El anillo vaginal debe insertarse en el día correcto de su ciclo mensual (ver sección 3.3 “Cuándo empezar con el primer anillo”) y permanecer en la vagina durante 3 semanas seguidas. Compruebe regularmente si el anillo está todavía en su vagina (por ejemplo antes y después de las relaciones sexuales) para asegurarse de que está protegida frente a un embarazo. Después de la tercera semana, quítese Ornibel y deje pasar una semana de descanso. Normalmente tendrá su periodo mensual o regla durante esta pausa sin anillo.
Mientras esté utilizando Ornibel no debe usar algunos métodos anticonceptivos de barrera femeninos, tales como el diafragma vaginal, el capuchón cervical o el condón femenino. Estos métodos anticonceptivos de barrera no deben usarse como método anticonceptivo de refuerzo de control de la natalidad ya que Ornibel puede interferir con la colocación y posición correctas del diafragma, capuchón cervical o condón femenino. Sin embargo, puede usar un condón masculino como método anticonceptivo de barrera adicional.
3.1 Forma de ponerse y quitarse el Ornibel
- Antes de ponerse el anillo, compruebe que no esté caducado (vea sección 5 “Conservación de Ornibel”).
- Lávese las manos antes de ponerse o quitarse el anillo.
- Escoja la postura que sea más cómoda para usted para insertárselo, por ejemplo de pie con una pierna levantada hacia arriba, en cuclillas o tumbada.
- Retire Ornibel de su sobre. Guarde el sobre para un uso posterior.
- Sujete el anillo entre los dedos índice y pulgar, presione los lados opuestos e introduzca el anillo en la vagina (ver figuras 1-4).
Cuando Ornibel esté en su sitio no debe notar nada. Si se siente incómoda empuje suavemente Ornibel al interior de la vagina. La posición exacta del anillo en el interior de la vagina no es importante.
- Pasadas 3 semanas, quítese el Ornibel de la vagina. Puede hacerlo enganchando el anillo con el dedo índice o sujetando con los dedos y estirando hacia fuera (ver figura 5). En caso de que no pueda quitarse el anillo a pesar de haberlo localizado, contacte con su médico.
- Elimine el anillo usado con la basura normal de la casa, preferiblemente en su sobre. No tire Ornibel al inodoro.
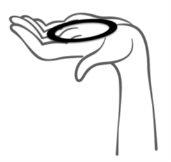
Figura 1
Retire Ornibel de su sobre
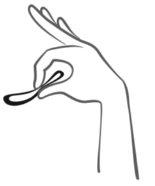
Figura 2
Presione el anillo
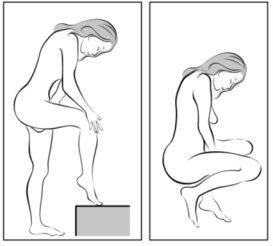
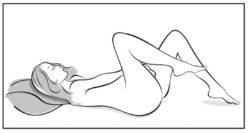
Figura 3
Escoja una posisición cómoda para ponerse el anillo
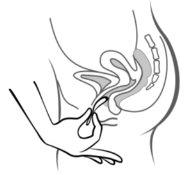
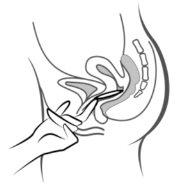
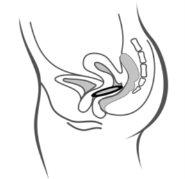
Figura 4A Figura 4B Figura 4C
Póngase el anillo en la vagina con una mano (Figura 4A), si es necesario separe los labios de la vagina con la otra. Empuje el anillo al interior de la vagina hasta que lo sienta cómodo (Figura 4B). Deje el anillo en la vagina durante 3 semanas (Figura 4C).
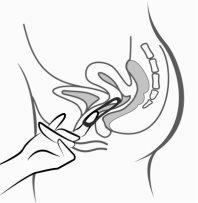
Figura 5:
Ornibel puede retirarse enganchando el anillo con el dedo índice o sujetando entre el índice y el dedo medio y estirando hacia fuera.
3.2 Tres semanas dentro, una semana fuera
- El anillo vaginal debe permanecer en la vagina desde el día en que se lo pone durante 3 semanas seguidas, ininterrumpidamente.
- Al cabo de 3 semanas quítese el anillo el mismo día de la semana que se lo puso y a la misma hora aproximadamente. Por ejemplo, si se lo puso un miércoles aproximadamente a las 22.00 h, debe quitárselo 3 semanas más tarde, en miércoles, aproximadamente a las 22.00 h.
- Una vez que se haya quitado el anillo, no lo use durante una semana. Durante esta semana debe producirse un sangrado vaginal. Normalmente empieza 2-3 días después de quitarse el Ornibel.
- Póngase un nuevo anillo exactamente después del intervalo de 1 semana (de nuevo el mismo día de la semana y aproximadamente a la misma hora), incluso si aún no ha dejado de sangrar.
Si se pone el nuevo anillo con más de 3 horas de retraso, la eficacia anticonceptiva puede reducirse. Siga las instrucciones de la sección 3.4 “Qué hacer si olvida ponerse un nuevo anillo después de la pausa sin anillo”.
Si usa Ornibel como se ha descrito más arriba, su sangrado tendrá lugar aproximadamente los mismos días cada mes.
3.3 Cuándo empezar con el primer anillo
- Si no ha usado anticoncepción hormonal en el mes anterior
Póngase Ornibel el primer día de su ciclo natural (es decir, el primer día de su menstruación). Ornibel empieza a actuar enseguida. No necesita tomar otras medidas anticonceptivas.
También se puede empezar con Ornibel entre el día 2 y el 5 de su ciclo, pero en caso de que tenga relaciones sexuales durante los primeros 7 días de uso de Ornibel, asegúrese de utilizar un método anticonceptivo complementario (preservativo). Sólo debe seguir esta recomendación cuando use Ornibel por primera vez.
- Si usaba una píldora combinada en el mes anterior
Empiece a usar Ornibel como más tarde el día siguiente después de la pausa con su píldora actual. Si el envase de su píldora también contiene comprimidos inactivos, empiece Ornibel como más tarde al día siguiente del último comprimido inactivo. Si no está segura de qué comprimido es, consúltelo a su médico o a su farmacéutico. No alargue nunca los días de descanso de su píldora actual más allá de lo recomendado.
Si ha tomado su píldora actual de forma continuada y correcta y está segura de que no está embarazada, también puede dejar de tomar la píldora cualquier día de su ciclo actual y empezar con Ornibel inmediatamente.
- Si usaba un parche transdérmico el mes anterior
Empiece a usar Ornibel como más tarde el día siguiente después de la pausa sin parche. No alargue los días de descanso sin parche más allá de lo recomendado.
Si ha utilizado el parche de forma continuada y correcta y está segura de que no está embarazada, también puede dejar el parche cualquier día y empezar con Ornibel inmediatamente.
- Si usaba una píldora con progestágeno solo en el mes anterior
Puede dejar de tomar su píldora con progestágeno solo cualquier día y empezar Ornibel al día siguiente a la misma hora. Pero asegúrese de que también usa un método anticonceptivo complementario (preservativo) durante los primeros 7 días de usar el anillo.
- Si usaba un inyectable, un implante o un Sistema de Liberación Intrauterino [SLI] con carga hormonal (progestágeno) en el mes anterior
Empiece a usar Ornibel en el momento en que debería recibir la siguiente inyección o el día que le extraigan el implante o el SLI. Pero asegúrese de utilizar un método anticonceptivo complementario (un preservativo) durante los primeros 7 días de usar el anillo.
- Después del parto
Si acaba de tener un niño, su médico le puede aconsejar que espere hasta que aparezca su primera regla normal antes de empezar con Ornibel. A veces es posible empezar antes, su médico le aconsejará cómo hacerlo. Si está dando el pecho y desea utilizar Ornibel debe consultarlo primero con su médico.
- Después de un aborto
Consulte a su médico.
3.4 Qué hacer si...
Su anillo se expulsa accidentalmente de la vagina
Ornibel puede expulsarse accidentalmente fuera de la vagina, por ejemplo si no se lo ha insertado adecuadamente, al quitarse un tampón, durante las relaciones sexuales, si tiene estreñimiento o si tiene prolapso uterino (descenso de la matriz). Por ello, debe comprobar regularmente si el anillo está en su vagina. (Por ejemplo, antes y después de mantener relaciones sexuales).
Su anillo ha estado fuera de la vagina temporalmente
Ornibel aún podría protegerle de quedarse embarazada, pero esto dependerá de cuánto tiempo haya estado fuera de su vagina.
Si el anillo ha estado fuera de la vagina durante:
- Menos de 3 horas, todavía la protegerá del embarazo. Debe lavar el anillo con agua fría o tibia (no utilice agua caliente) y volver a colocarse el anillo en la vagina lo antes posible, pero sólo si el anillo ha estado fuera de la vagina menos de 3 horas.
- Más de 3 horas en la 1ª ó 2ª semana, podría no protegerle del embarazo. Debe lavar el anillo con agua fría o tibia (no use agua caliente) y póngase el anillo tan pronto como se acuerde y déjelo en la vagina durante al menos 7 días seguidos. Use un preservativo masculino si mantiene relaciones sexuales durante esos 7 días. Si está en la primera semana, y ha mantenido relaciones sexuales en los 7 días anteriores, existe la posibilidad de que estuviese embarazada. En ese caso, consulte a su médico.
- Más de 3 horas en la 3ª semana, podría no protegerla del embarazo. Debe quitarse el anillo y escoger una de las dos opciones siguientes:
- Póngase un nuevo anillo inmediatamente.
Al ponerse un nuevo anillo iniciará un nuevo ciclo de uso de tres semanas y puede ser que no aparezca el periodo. Sin embargo, puede producirse sangrado intermenstrual o manchado durante ese ciclo.
- No se ponga un nuevo anillo. Deje pasar el sangrado intermenstrual y póngase un nuevo anillo no más tarde de 7 días desde el momento en que el anillo anterior se quitó o se expulsó.
Escoja esta opción sólo si ha utilizado Ornibel de forma seguida durante los 7 días anteriores.
Si Ornibel ha estado fuera de la vagina por un periodo de tiempo desconocido, puede no estar protegida frente a un embarazo. Deberá realizarse un test de embarazo y consultar a su médico antes de insertar un nuevo anillo
El anillo se abre
Ornibel pueda abrirse. Se ha notificado lesión vaginal asociada a la rotura del anillo. Si nota que Ornibel se ha abierto, quítese el anillo y póngase uno nuevo tan pronto como sea posible. Tome precauciones anticonceptivas complementarias (por ejemplo un preservativo masculino) durante los 7 días siguientes. Si ha tenido relaciones sexuales antes de notar que el anillo se había abierto, consulte con su médico.
Se ha puesto más de un anillo
No hay informes de daños graves debido a sobredosis de las hormonas de Ornibel. Si accidentalmente se ha puesto más de un anillo, puede sentirse mal (náuseas), vomitar o tener un sangrado vaginal. Quítese el anillo de más y contacte con su médico si estos síntomas no desaparecen. También puede llamar al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad utilizada.
Si olvida ponerse un nuevo anillo después de la pausa sin anillo
Su pausa sin anilloha durado más de 7 días. Póngase un nuevo anillo en la vagina tan pronto como lo recuerde. Tome medidas anticonceptivas adicionales (como el preservativo masculino) si mantiene relaciones sexuales durante los siguientes 7 días. Si ha tenido relaciones sexuales en esta pausa sin anillo existe la posibilidad de que se hubiera quedado embarazada. En tal caso, informe a su médico inmediatamente. Cuanto más larga sea la pausa sin anillo, el riesgo de quedarse embarazada aumenta.
Si ha olvidado quitarse el anillo
- Si el anillo ha estado en la vagina entre 3 y 4 semanas, todavía la protegerá del embarazo. Deje pasar la semana sin anillo y después póngase uno nuevo.
- Si el anillo ha estado en la vagina durante más de 4 semanas, existe la posibilidad de embarazo. Contacte con su médico antes de empezar con un nuevo anillo.
Si no aparece el periodo o regla
- Ha usado Ornibel según las instrucciones.
Si no aparece el periodo, pero ha usado Ornibel según las instrucciones y no ha tomado otros medicamentos, es muy improbable que esté embarazada. Continúe usando Ornibel de la forma habitual. Sin embargo, si tiene dos faltas seguidas puede estar embarazada, por lo que debe comunicárselo a su médico inmediatamente. No se ponga el siguiente Ornibel hasta que su médico haya comprobado que no está embarazada.
- Si se ha desviado de las recomendaciones de uso de Ornibel.
Si no se presentase su sangrado habitual en la pausa de una semana sin anillo y se ha desviado del régimen recomendado, existe la posibilidad de que pueda estar embarazada, por lo que deberá contactar con su médico antes de ponerse un nuevo anillo.
Si tiene un sangrado inesperado
En algunas mujeres, durante el uso de Ornibel puede aparecer un sangrado vaginal inesperado entre sus periodos menstruales. Puede necesitar protección higiénica. De todas formas, continúe utilizando el anillo de la forma normal, no se lo quite. Si el sangrado continúa, se hace más intenso o empieza de nuevo, consulte a su médico.
Si desea cambiar el día en que empieza su periodo o regla
Si usa Ornibel según las instrucciones, su periodo menstrual (sangrado por deprivación) empezará en la semana de pausa sin anillo. Si quiere cambiar el día que empieza, tiene que acortar (¡nunca alargar!) la pausa sin anillo.
Por ejemplo, si su periodo suele empezar un viernes, puede cambiarlo a un martes, es decir 3 días antes a partir del mes siguiente. Simplemente, póngase el siguiente anillo tres días antes del día habitual.
Si la pausa se hace muy corta (por ejemplo 3 días o menos), puede ser que no tenga su sangrado habitual. Puede tener un manchado (gotas o manchas de sangre) o sangrado intermenstrual durante el uso del siguiente anillo.
Si no está segura de cómo hacerlo, consulte a su médico.
Si desea retrasar su periodo o regla
Aunque no es el régimen recomendado, puede retrasar su periodo (sangrado por deprivación) poniéndose un nuevo anillo inmediatamente después de quitarse el anillo actual, sin dejar la pausa entre anillos. Puede dejarse puesto el nuevo anillo durante 3 semanas como máximo. Durante el uso del nuevo anillo, puede experimentar manchado (gotas o manchas de sangre) o sangrado irregular. Cuando desee que empiece su periodo simplemente quítese el anillo. Deje la pausa normal de una semana sin anillo y póngase después un anillo nuevo.
Puede consultar a su médico antes de decidir retrasar su periodo menstrual.
3.5 Si desea dejar de usar Ornibel
Puede dejar de utilizar Ornibel en cualquier momento que lo desee. Si no desea quedar embarazada consulte a su médico sobre otros métodos anticonceptivos.
Si deja de utilizar Ornibel porque desea quedarse embarazada, se recomienda esperar hasta que haya tenido su primera regla natural antes de intentar concebir. Esto le ayudará a calcular la fecha del parto.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Si sufre cualquier efecto adverso, especialmente si es grave y persistente, o tiene algún cambio de salud que cree que puede deberse a Ornibel, consulte a su médico.
Todas las mujeres que toman anticonceptivos hormonales combinados corren mayor riesgo de presentar coágulos de sangre en las venas (tromboembolismo venoso (TEV)) o coágulos de sangre en las arterias (tromboembolismo arterial (TEA)). Para obtener información más detallada sobre los diferentes riesgos de tomar anticonceptivos hormonales combinados, ver sección 2 “Qué necesita saber antes de empezar a usar Ornibel”.
Contacte con un médico de forma inmediata si experimenta cualquiera de los siguientes síntomas de angioedema: hinchazón de la cara, lengua y/o garganta y/o dificultad para tragar o urticaria con posible dificultad para respirar (ver también sección “Advertencias y precauciones”).
Usuarias del anillo conteniendo Etonogestrel / Etinilestradiol han informado de los siguientes efectos adversos:
Frecuentes:pueden afectar hasta 1 de cada 10 mujeres.
- dolor abdominal, malestar (náuseas)
- infección de la vagina por levaduras (parecido a candidiasis oral), molestias vaginales debidas al anillo, picor genital, flujo vaginal
- dolor de cabeza o migraña, humor depresivo, menos deseo sexual
- dolor en las mamas, dolor en la pelvis, menstruación dolorosa
- acné
- aumento de peso
- expulsión del anillo
Poco frecuentes:pueden afectar hasta 1 de cada 100 mujeres.
- alteraciones de la vista, mareos
- abdomen hinchado, vómitos, diarreas o estreñimiento
- sensación de estar cansada, molesta o irritable, cambios de humor, cambios en el estado de ánimo
- retención de líquidos en el cuerpo (edema)
- infección de orina o de la vejiga
- dificultad, dolor al orinar; urgencia de orinar. Necesidad de orinar más frecuente
- problemas en las relaciones sexuales como dolor, sangrado o que la pareja note el anillo
- aumento de la presión sanguínea
- aumento del apetito
- dolor de espalda, calambres en los músculos, dolor en las piernas o brazos
- menos sensibilidad en la piel
- tensión o dolor en las mamas o aumento de tamaño; enfermedad fibroquística de a mama (quistes en las mamas que pueden llegar a hincharse o doler)
- inflamación del cuello del útero, pólipos que crecen en el cuello del útero, desplazamiento hacia la parte exterior de tejido del interior del cuello del útero (ectropión)
- cambios en el periodo menstrual (más intensos, largos, irregulares o desaparecer), molestias en la pelvis, síndrome premenstrual, calambres uterinos
- infección vaginal (por hongos o bacterias), quemazón, olor, dolor, molestias o sequedad de la vagina o vulva
- pérdida de pelo, eczema, picor, erupción o sofocos
- Urticaria
Raros:pueden afectar hasta 1 de cada 1.000 mujeres.
- coágulos de sangre perjudiciales en una vena o arteria, por ejemplo:
- en una pierna o pie (es decir, TVP)
- en un pulmón (es decir, EP)
- ataque al corazón
- ictus
- ictus leve o síntomas temporales similares a los de un ictus, lo que se llama accidente isquémico transitorio (AIT)
- coágulos de sangre en el hígado, estómago/intestino, riñones u ojo
Las posibilidades de tener un coágulo de sangre pueden ser mayores si tiene cualquier otra afección que aumente este riesgo (ver sección 2 para obtener más información sobre las afecciones que aumentan el riesgo de padecer coágulos de sangre y los síntomas de un coágulo de sangre).
- secreción de las mamas
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles).
- Cloasma (manchas de color amarillento-marrón en la piel, particularmente en la cara)
- Molestias en el pene de la pareja (irritación, erupción, picor).
- Incapacidad para quitarse el anillo sin asistencia médica (p.ej. debido a su adherencia a la pared vagianal).
- Lesión vaginal asociada a la rotura del anillo.
Se han notificado casos de cáncer de mama y tumores en el hígado en mujeres que utilizan anticonceptivos hormonales combinados. Para más información ver la sección 2.2 Advertencias y precauciones, Cáncer.
Ornibel puede abrirse. Para más información, véase sección 3.4 Qué hacer si…el anillo se abre.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es
5. Conservación de Ornibel
Mantener este medicamento fuera de la vista y del alcance de los niños.
Si descubre que un niño se ha expuesto a las hormonas de Ornibel, pida consejo a su médico.
Este medicamento no requiere ninguna temperatura especial de conservación.
Conservar en el embalaje original para protegerlo de la luz.
Ornibel debe insertarse al menos un mes antes de su fecha de caducidad aparece en la caja y en cada sobre después de CAD. La fecha de caducidad es el último dia del mes que se indica.
No utilice Ornibel si observa cambios en el color del anillo o cualquier signo visible de deterioro.
Este medicamento puede suponer un riesgo para el medio ambiente. Una vez retirado, Ornibel debe volverse a guardar en su sobre y cerrarlo adecuadamente. El sobre cerrado debe desecharse con los residuos normales de la casa o debe devolverse a la farmacia para una destrucción adecuada conforme a la normativa local.
No debe tirarse por el inodoro. Al igual que con otros medicamentos, no tire los anillos no utilizados o caducados por los desagües ni a la basura.
Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ornibel
- Los principios activos son etonogestrel y etinilestradiol.
Ornibel contiene 8,25 mg de etonogestrel y 2,60 mg de etinilestradiol. El anillo libera etonogestrel y etinilestradiol en una cantidad de 0,120 mg y 0,015 mg, respectivamente durante 24 horas, por un periodo de 3 semanas.
- Los demás componentes son: copolímero de acetato de vinilo y etileno 28% acetato de vinilo y poliuretano (un tipo de plástico que no se disuelve en el cuerpo).
Aspecto de Ornibel y contenido del envase
Sistema de liberación vaginal.
Ornibel es un anillo flexible, transparente, incoloro o casi incoloro, que mide 54 mm de diametro y 4 mm de sección.
Cada anillo se envasa en un sobre de aluminio. El sobre se presenta en una caja de cartón junto a este prospecto y pegatinas para su calendario que le ayuden a recordar cuando insertar o retirar el anillo.
Cada caja contiene 1, 3 o 6 anillos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Exeltis Healthcare, S.L.
Avda. De Miralcampo 7.
Polígono Industrial Miralcampo.
19200 Azuqueca de Henares. Guadalajara.
España
Responsable de la fabricación:
Laboratorios León Farma, S,A.
Pol. Ind. Navatejera.
La Vallina s/n.
24193 Villaquilambre, León.
España.
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con el siguiente nombre:
Fecha de la última revisión de este prospecto:Noviembre 2024
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/)
- País de registro
- Precio medio en farmacia9.9 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ORNIBEL 0,120 MG/0,015 MG CADA 24 HORAS SISTEMA DE LIBERACION VAGINAL EFGForma farmacéutica: DISPOSITIVO VAGINAL, 0,120 mg/0,015 mg cada 24 horasPrincipio activo: vaginal ring with progestogen and estrogenFabricante: Organon Salud S.L.Requiere recetaForma farmacéutica: DISPOSITIVO VAGINAL, 0,120 mg/0,015 mgPrincipio activo: vaginal ring with progestogen and estrogenFabricante: Laboratorios Cinfa S.A.Requiere recetaForma farmacéutica: DISPOSITIVO VAGINAL, 11,7 mg etonogestrel/ 2,7 mg etinilestradiolPrincipio activo: vaginal ring with progestogen and estrogenFabricante: Organon Salud S.L.Requiere receta
Médicos online para ORNIBEL 0,120 MG/0,015 MG CADA 24 HORAS SISTEMA DE LIBERACION VAGINAL EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ORNIBEL 0,120 MG/0,015 MG CADA 24 HORAS SISTEMA DE LIBERACION VAGINAL EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






