
ONNIMIA 250 MG SOLUCIÓN INYECTABLE EN JERINGA PRECARGADA EFG

Cómo usar ONNIMIA 250 MG SOLUCIÓN INYECTABLE EN JERINGA PRECARGADA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Onnimia 250 mg solución inyectable en jeringa precargada EFG
fulvestrant
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Onnimia y para qué se utiliza
- Qué necesita saber antes de empezar a usar Onnimia
- Cómo usar Onnimia
- Posibles efectos adversos
- Conservación de Onnimia
- Contenido del envase e información adicional
1. Qué es Onnimia y para qué se utiliza
Onnimia contiene el principio activo fulvestrant, que pertenece al grupo de bloqueantes de estrógeno. Los estrógenos, un tipo de hormonas sexuales femeninas, pueden estar en algunos casos implicados en el desarrollo del cáncer de mama.
Fulvestrant se utiliza:
? en monoterapia, para tratar mujeres posmenopáusicas con un tipo de cáncer de mama llamado cáncer de mama con receptor de estrógeno positivo, que es localmente avanzado o que se ha extendido a otras partes del cuerpo (metastásico), o
? en combinación con palbociclib para tratar a mujeres con un tipo de cáncer de mama llamado cáncer de mama con receptor hormonal positivo, cáncer de mama con receptor 2 del factor de crecimiento epidérmico humano negativo, que está localmente avanzado o que se ha extendido a otras partes del cuerpo (metastásico). Las mujeres que no hayan llegado a la menopausia también serán tratadas con un medicamento llamado agonista de la hormona liberadora de hormona luteinizante (LHRH).
Fulvestrant se puede administrar en combinación con palbociclib. Es importante que usted lea también el prospecto de palbociclib. Si tiene alguna pregunta sobre palbociclib, consulte a su médico.
2. Qué necesita saber antes de empezar a usar Onnimia
No use Onnimia:
- si es alérgica a fulvestrant o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si está embarazada o en periodo de lactancia
- si presenta problemas hepáticos graves
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Onnimia si algo de esto le aplica:
- problemas de riñón o hígado
- recuento bajo de plaquetas (que ayudan a la coagulación de la sangre) o alteraciones hemorrágicas
- problemas previos de coágulos sanguíneos
- osteoporosis (pérdida de densidad ósea)
- alcoholismo
Niños y adolescentes
Onnimia no está indicado en niños y adolescentes menores de 18 años.
Uso de Onnimia con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
En particular, debe decir a su médico si está utilizando anticoagulantes (medicamentos para prevenir los coágulos sanguíneos).
Embarazo y lactancia
No debe utilizar fulvestrant si está usted embarazada. Si puede quedarse embarazada, debe utilizar un método anticonceptivo eficaz mientras esté en tratamiento con Onnimia y durante dos años después de última dosis.
No debe dar el pecho mientras esté en tratamiento con fulvestrant.
Conducción y uso de máquinas
No se espera que fulvestrant afecte a su capacidad para conducir o utilizar máquinas. Sin embargo, si se siente cansada después del tratamiento no conduzca ni utilice máquinas.
Uso en deportistas
Este medicamento contiene fulvestrant que puede producir un resultado positivo en las pruebas de control de dopaje.
Onnimia contiene etanol (alcohol), alcohol bencílico, benzoato de bencilo y aceite de ricino refinado.
Este medicamento contiene un 10% p/v de etanol (alcohol), que se corresponde con una cantidad de 500 mg por dosis, lo que equivale a 10 ml de cerveza o 4 ml de vino. Este medicamento es perjudicial para personas que padecen alcoholismo.
El contenido en alcohol debe tenerse en cuenta en el caso de mujeres embarazadas o en período de lactancia, niños y grupos de alto riesgo, como pacientes con enfermedades del hígado o epilepsia.
Este medicamento contiene 500 mg de alcohol bencílico por inyección, lo que equivale a 100 mg/ml.
El alcohol bencílico puede provocar reacciones alérgicas.
Consulte a su médico o farmacéutico si está embarazada o en período de lactancia o si tiene enfermedades de hígado o riñón. Esto es debido a que se pueden acumular grandes cantidades de alcohol bencílico en su organismo y provocar efectos adversos (lo que se denomina “acidosis metabólica”).
Este medicamento contiene 750 mg de benzoato de bencilo por inyección, lo que equivale a 150 mg/ml.
Este medicamento puede producir reacciones alérgicas graves porque contiene aceite de ricino refinado.
3. Cómo usar Onnimia
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es 500 mg de fulvestrant (dos inyecciones de 250 mg/5 ml) administrada una vez al mes con una dosis adicional de 500 mg administrada 2 semanas después de la dosis inicial.
Su médico o enfermero le administrará fulvestrant mediante una inyección intramuscular lenta en cada uno de sus glúteos.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Puede necesitar tratamiento médico urgente si experimenta alguno de los siguientes efectos adversos:
? Reacciones alérgicas (hipersensibilidad), incluyendo hinchazón de la cara, labios, lengua y/o garganta, que pueden ser síntomas de reacciones anafilácticas
? Tromboembolismo (aumento del riesgo de coágulos sanguíneos)*
? Inflamación del hígado (hepatitis)
? Fallo hepático
Informe inmediatamente a su médico, farmacéutico o enfermero si nota alguno de los siguientes efectos adversos:
Efectos adversos muy frecuentes(puede afectar a más de 1 de cada 10 personas)
? Reacciones en el lugar de la inyección, como dolor y/o inflamación
? Niveles anormales de enzimas hepáticos (en análisis de sangre)*
? Náuseas (sensación de malestar)
? Debilidad, cansancio*
? Dolor articular y musculoesquelético
? Sofocos
? Erupción cutánea
? Reacciones alérgicas (hipersensibilidad), incluyendo hinchazón de la cara, labios, lengua y/o garganta.
Todos los efectos adversos restantes:
Efectos adversos frecuentes(puede afectar hasta a 1 de cada 10 personas)
? Dolor de cabeza
? Vómitos, diarrea o pérdida del apetito*
? Infecciones del tracto urinario
? Dolor de espalda*
? Aumento de bilirrubina (un pigmento de la bilis producido por el hígado)
? Tromboembolismo (aumento del riesgo de coágulos sanguíneos)*
? Niveles disminuidos de plaquetas (trombocitopenia)
? Hemorragia vaginal
? Dolor lumbar que se refleja en un lado de la pierna (ciática)
? Debilidad repentina, entumecimiento, hormigueo o pérdida de movimiento en su pierna, especialmente en un solo lado del cuerpo, problemas repentinos para caminar o de equilibrio (neuropatía periférica).
Efectos adversos poco frecuentes(puede afectar hasta a 1 de cada 100 personas)
? Flujo vaginal espeso, blanquecino y candidiasis (infección)
? Hematoma y hemorragia en el lugar de la inyección
? Aumento de gamma-GT, un enzima hepático que se identifica en un análisis de sangre
? Inflamación del hígado (hepatitis)
? Fallo hepático
? Entumecimiento, hormigueo y dolor
? Reacciones anafilácticas.
- Incluye efectos adversos para los cuales no se puede evaluar el papel exacto de fulvestrant debido a la enfermedad subyacente.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es/ . Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Onnimia
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase o en las etiquetas de las jeringas después de la abreviatura CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar y transportar refrigerado (entre 2ºC y 8ºC).
Las desviaciones de temperatura fuera del rango de entre 2ºC y 8ºC deben ser controladas. Esto incluye evitar la conservación a temperaturas superiores a 30ºC, y que no exceda un periodo de 28 días, durante el cual la temperatura media de conservación del medicamento sea inferior a 25ºC (pero por encima de entre 2ºC y 8ºC). Tras las desviaciones de temperatura, el medicamento debe ser retornado de forma inmediata a las condiciones de conservación recomendadas (conservar y transportar refrigerado entre 2ºC y 8ºC). Las desviaciones de temperatura tienen un efecto acumulativo en la calidad del medicamento, no debiéndose superar el periodo de 28 días por encima de la duración de la caducidad de 2 años de Onnimia La exposición a temperaturas inferiores a 2ºC no dañará el medicamento, siempre y cuando éste no se conserve por debajo de los -20ºC.
Conservar la jeringa precargada en el embalaje original para protegerla de la luz.
Su profesional sanitario será el responsable de la conservación, uso y eliminación correctos de Onnimia.
Este medicamento puede presentar un riesgo para el medio acuático. Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido de Onnimia
Composición de Onnimia
- El principio activo es fulvestrant. Cada jeringa precargada (5 ml) contiene 250 mg de fulvestrant. Cada ml contiene 50 mg de fulvestrant.
- Los demás componentes (excipientes) son etanol (96 por ciento), alcohol bencílico, benzoato de bencilo y aceite de ricino refinado.
Aspecto del producto y contenido del envase
Onnimia es una solución viscosa, transparente, de incolora a amarilla en una jeringa precargada equipada con un cierre a prueba de manipulación, que contiene 5 ml de solución inyectable. Deben administrarse dos jeringas para recibir la dosis mensual recomendada de 500 mg.
Onnimia presenta 2 formatos, bien un envase que contiene 1 jeringa de vidrio precargada o bien un envase que contiene 2 jeringas de vidrio precargadas. Se proporcionan además agujas con sistema de seguridad (“BD SafetyGlide”) para su conexión al cuerpo de cada jeringa.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Adamed Laboratorios, S.L.U.
c/ de las Rosas de Aravaca, 31 - 2ª planta
28023 Aravaca - Madrid
España
Responsable de la fabricación
Laboratori FUNDACIO DAU
C/De la letra C, 12-14, Polígono Industrial de la Zona Franca
Barcelona 08040
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Francia | Fulvestrant Intas 250 mg, solution injectable en seringue préremplie |
Alemania | Fulvestrant Intas 250 mg Injektionslösung in einer Fertigspritze. |
España | Onnimia 250 mg solución inyectable en jeringa precargada EFG |
Fecha de la última revisión de este prospecto:Agosto 2020
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
--------------------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Onnimia 500 mg (2 x 250 mg/5 ml solución inyectable) debe administrarse empleando dos jeringas precargadas, ver sección 3.
Instrucciones de administración
Advertencia – No esterilizar en autoclave la aguja con sistema de seguridad (Aguja Hipodérmica Protegida “BD SafetyGlide”) antes de su uso. Las manos deben permanecer por detrás de la aguja en todo momento durante su uso y eliminación.
Para cada una de las dos jeringas:
? Retire el cuerpo de vidrio de la jeringa de la bandeja y compruebe que no está dañado. ? Abra el envase exterior de la aguja con sistema de seguridad (“SafetyGlide”). ? Antes de su administración, se deben inspeccionar visualmente las soluciones parenterales en cuanto al contenido en partículas y a la decoloración. ? Mantenga la jeringa en posición vertical sujetándola por la parte estriada (C). Con la otra mano, sujete el tapón (A) e inclínelo cuidadosamente hacia atrás y adelante hasta que se desprenda la tapa y se pueda sacar, no la gire (ver Figura 1). | Figura 1
|
? Retire el tapón (A) tirando hacia arriba. Para mantener la esterilidad evite tocar la punta de la jeringa (B) (ver Figura 2) | Figura 2
|
? Acople la aguja con sistema de seguridad al “Luer-Lok” y enrósquela hasta que se acople firmemente (ver Figura 3). ? Compruebe que la aguja está acoplada al conector Luer antes de dejar de mantenerlo en posición vertical. ? Tire del capuchón protector de la aguja en línea recta para no dañar el extremo de la misma. ? Lleve la jeringa cargada al punto de administración. ? Retire el capuchón protector de la aguja. ? Elimine el exceso de gas de la jeringa. | Figura 3
|
? Administre lentamente por vía intramuscular en el glúteo (zona glútea) (1-2 minutos/inyección). Para una mayor comodidad, la posición de la aguja con el bisel hacia arriba tiene la misma orientación que el brazo de la palanca levantado (ver Figura 4). | Figura 4
|
? Tras la inyección, dé inmediatamente un solo toque con el dedo en el brazo de la palanca para activar el mecanismo de protección (ver Figura 5). NOTA: Active alejado de su cuerpo y de los demás. Escuche el clic y confirme visualmente que la punta de la aguja está totalmente protegida. |
|
Eliminación
Las jeringas precargadas son solopara un único uso.
Este medicamento puede presentar un riesgo para el medio acuático. La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Precio medio en farmacia408.86 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ONNIMIA 250 MG SOLUCIÓN INYECTABLE EN JERINGA PRECARGADA EFGForma farmacéutica: INYECTABLE, 250 mg/5 mlPrincipio activo: FulvestrantFabricante: Bexal Farmaceutica S.A.Requiere recetaForma farmacéutica: INYECTABLE, 250 mgPrincipio activo: FulvestrantFabricante: Ever Valinject GmbhRequiere recetaForma farmacéutica: INYECTABLE, 250 mgPrincipio activo: FulvestrantFabricante: Astrazeneca AbRequiere receta
Médicos online para ONNIMIA 250 MG SOLUCIÓN INYECTABLE EN JERINGA PRECARGADA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ONNIMIA 250 MG SOLUCIÓN INYECTABLE EN JERINGA PRECARGADA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes





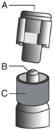
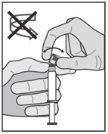
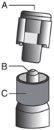
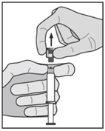
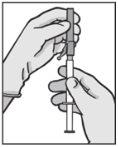
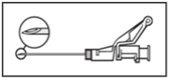
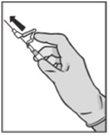 Figura 5
Figura 5







