NUCALA 100 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar NUCALA 100 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el paciente
Nucala 100mgsolución inyectable en jeringa precargada
mepolizumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Nucala y para qué se utiliza
- Qué necesita saber antes de empezar a usar Nucala
- Cómo usar Nucala
- Posibles efectos adversos
- Conservación de Nucala
- Contenido del envase e información adicional
- Instrucciones de uso paso a paso
1. Qué es Nucala y para qué se utiliza
Nucala contiene la sustancia activa mepolizumab, un anticuerpo monoclonal, un tipo de proteína diseñada para reconocer una sustancia diana específica en el cuerpo. Se utiliza para tratar el asma grave y la GEPA(Granulomatosis Eosinofílica con Poliangeítis) en adultos, adolescentes y niños a partir de 6 años. También se utiliza para tratar la RSCcPN(Rinosinusitis Crónica con Pólipos Nasales) y el SHE(Síndrome Hipereosinofílico) en adultos.
Mepolizumab, la sustancia activa de Nucala, bloquea una proteína llamada interleucina-5. Al bloquear la acción de esta proteína, se limita la producción de eosinófilos por la médula ósea y disminuye el número de eosinófilos en la sangre y los pulmones.
Asma eosinofílica grave
Algunas personas con asma grave tienen demasiados eosinófilos(un tipo de glóbulo blanco) en la sangre y los pulmones. Esta condición se llama asma eosinofílica– el tipo de asma que Nucala puede tratar.
Si usted o su hijo ya están usando medicamentos como inhaladores a dosis altas, pero su asma no está bien controlado por estos medicamentos, Nucala puede reducir el número de ataques de asma.Si está tomando medicamentos llamados corticosteroides orales, Nucala también puede ayudar a reducir la dosis diaria que necesita para controlar su asma.
Rinosinusitiscrónica con pólipos nasales (RSCcPN)
La RSCcPN es una enfermedad en la cual las personas tiene demasiados eosinófilos(un tipo de glóbulo blanco) en la sangre, en los tejidos que recubren la nariz y en los senos nasales. Esto puede producir síntomas tales como congestión nasal y pérdida de olfato, así como crecimientos blandos gelatinosos (llamados pólipos nasales) que se forman dentro de la nariz.
Nucala reduce el número de eosinófilos en la sangre y puede reducir el tamaño de sus pólipos, aliviando la congestión nasal y ayudando a prevenir la cirugía de pólipos nasales.
Nucala también puede ayudar a reducir la necesidad de corticosteroides oralespara controlar sus síntomas.
Granulomatosis eosinofílica con poliangeítis(GEPA)
GEPA es una enfermedad en la que las personas tienen demasiados eosinófilos(un tipo de glóbulo blanco) en la sangre y los tejidos y además tienen alguna forma de vasculitis. Esto significa que hay inflamación en los vasos sanguíneos. Esta enfermedad afecta más comúnmente a los pulmones y los senos nasales, pero a menudo afecta a otros órganos como la piel, el corazón y los riñones.
Nucala puede controlar o retrasar un brote de estos síntomas de GEPA. Este medicamento puede también ayudar a reducir la dosis diaria de corticosteroidesoralesque necesita para controlar sus síntomas.
Síndrome hipereosinofílico (SHE)
El síndrome hipereosinofílico (SHE) es una enfermedad en la que hay un elevado número de eosinófilos(un tipo de glóbulo blanco) en la sangre. Estas células pueden dañar los órganos del cuerpo, en particular el corazón, pulmones, nervios y la piel.
Nucala ayuda a reducir sus síntomas y previene los brotes. Si está tomando medicamentos conocidos comúnmente como corticosteroides orales, Nucala también puede ayudar a reducir la dosis diaria de estos, que necesita para controlar sus síntomas y brotes de SHE.
2. Qué necesita saber antes de empezar a usar Nucala
No use Nucala:
- si es alérgicoa mepolizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Consulte con su médicosi piensa que esto le aplica.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Nucala.
Empeoramiento del asma
Algunas personas tienen efectos adversos relacionados con el asma, o su asma puede empeorar durante el tratamiento con Nucala.
- Consulte con su médico o enfermerosi el asma permanece no controlado, o empeora, tras comenzar el tratamiento con Nucala.
Reacciones alérgicas y en el lugar de la inyección
Los medicamentos de este tipo (anticuerpos monoclonales)pueden causar reacciones alérgicas graves cuando se inyectan en el cuerpo (ver sección 4, “Posibles efectos adversos”).
Si ha tenido alguna vez una reacción similar a cualquier inyección o medicamento:
- Consulte con su médicoantes de que le administren Nucala.
Infecciones parasitarias
Nucala puede debilitar su resistencia a las infecciones causadas por parásitos. Si ya tiene una infección parasitaria, se debe tratar antes de iniciar el tratamiento con Nucala. Si vive en una zona donde estas infecciones son comunes o si va a viajar a una de estas zonas:
- Consulte con su médicosi piensa que alguna de estas circunstancias le aplican.
Niños y adolescentes
Asma eosinofílica grave
La jeringa precargada no está indicada para el uso en niños menores de 12 añospara el tratamiento del asma eosinofílica grave.
Para niños de 6-11 años, consulte con su médico que le prescribirá la dosis recomendada de Nucala que será administrada por un enfermero o médico.
RSCcPN
Este medicamento no está indicado para el uso en niños o adolescentes menores de 18 añospara el tratamiento de la RSCcPN.
GEPA
Este medicamento no está indicado para el uso en niños menores de 6 añospara el tratamiento de GEPA.
SHE
Este medicamento no está indicado para el uso en adolescentes o niños menores de 18 añospara el tratamiento del SHE.
Otros medicamentos y Nucala
Informe a su médicosi está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Otros medicamentos para el asma, RSCcPN, GEPA o SHE
- Una vez que haya comenzado el tratamiento con Nucala, no deje de tomarde forma repentina los medicamentos que venía tomando para prevenir su asma, RSCcPN, GEPA o SHE. Estos medicamentos (especialmente los llamados corticosteroides orales) se deben dejar de tomar gradualmente, bajo la supervisión directa de su médico y dependiendo de su respuesta a Nucala.
Embarazo y lactancia
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médicoantes de utilizar este medicamento.
Se desconoce si los componentes de Nucala pueden pasar a la leche materna. Si está en periodo de lactancia, debe consultar con su médicoantes de utilizar Nucala.
Conducción y uso de máquinas
Es poco probable que los posibles efectos secundarios de Nucala afecten a su capacidad para conducir o utilizar maquinaria.
Nucala contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por 100 mg; esto es, esencialmente “exento de sodio”.
3. Cómo usar Nucala
Nucala se administra mediante una inyección justo bajo su piel (inyección subcutánea).
Su médico o enfermero decidirá si usted o su cuidador pueden inyectar Nucala. Si lo consideran apropiado, usted o su cuidador recibirán el entrenamiento para utilizar Nucala de una forma correcta.
En niños entre 6 y 11 años de edad, Nucala debe ser administrado por el médico, la enfermera o un cuidador entrenado.
Asma eosinofílica grave
La dosis recomendadaen adultos y adolescentes a partir de 12 años es de 100 mg. Se administrará una inyección cada cuatro semanas.
RSCcPN
La dosis recomendadaen adultos es 100 mg. Se administrará 1 inyección cada cuatro semanas.
GEPA
La dosis recomendadaen adultos y adolescentes a partir de 12 años es de 300 mg. Se administrará 3 inyecciones cada cuatro semanas.
Niños de 6 a 11 años de edad
Niños que pesan40 kg o más:
La dosis recomendadaes de 200 mg. Se administrará 2 inyecciones cada cuatro semanas.
Niños que pesan menos de40 kg:
La dosis recomendadaes de 100 mg. Se administrará 1 inyección cada cuatro semanas.
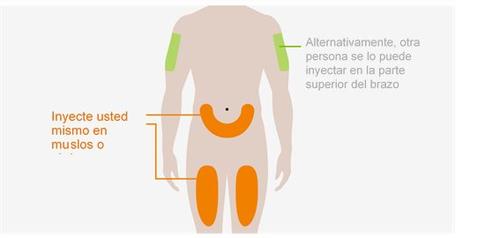
Los lugares de inyección deben estar al menos a 5 cm de distancia.
SHE
La dosis recomendadaen adultos y es de 300 mg. Se administrará 3 inyecciones cada cuatro semanas.
Los lugares de inyección deben estar al menos a 5 cm de distancia.
En el otro lado del prospecto puede encontrar las Instrucciones de uso de la jeringa precargada.
Si usa más Nucala del que debe
Consulte con su médicosi piensa que puede haberse administrado demasiado Nucala.
Si olvidó una dosis de Nucala
Usted o su cuidador deben inyectar la siguiente dosis de Nucala tan pronto como lo recuerden. Si no se da cuenta de que se ha olvidado una dosis hasta el momento en que le toca la siguiente, administre únicamente la siguiente dosis tal y como lo tenía planeado. Si no está seguro de qué hacer, pregunte a su médico, farmacéutico o enfermero.
Si interrumpe el tratamiento con Nucala
No deje de recibir inyecciones de Nucala a menos que su médico se lo indique. Interrumpir o detener el tratamiento con Nucala puede causar que sus síntomas y ataques vuelvan a aparecer.
Si sus síntomas empeoran mientras están administrándole inyecciones de Nucala:
- Avise a su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Los efectos adversos causados por Nucala son generalmente de leves a moderados, aunque ocasionalmente pueden ser graves.
Reacciones alérgicas
Algunas personas pueden tener reacciones alérgicas o de tipo alérgico. Estas reacciones pueden ser frecuentes (pueden afectar hasta a 1 de cada 10 personas). Por lo general ocurren en cuestión de minutos hasta horas después de la inyección, pero a veces los síntomas pueden comenzar incluso varios días después.
Los síntomas pueden incluir:
- opresión en el pecho, tos, dificultad para respirar
- desmayo, mareo, sensación de mareo (debido a una bajada de la tensión arterial)
- hinchazón de los párpados, la cara, los labios, la lengua o la boca
- habones
- erupción
- Si piensa que usted (o su hijo) está teniendo una reacción busque atención médica inmediatamente.
Si ha tenido alguna vez una reacción similar a cualquier inyección o medicamento:
- Consulte con su médicoantes de que a usted (o a su hijo) le administren Nucala.
Otros efectos adversos incluyen:
Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- dolor de cabeza
Frecuentes:pueden afectar hasta a 1 de cada 10 personas
- infección de pecho cuyos síntomas pueden incluir tos y fiebre (temperatura elevada)
- infección del tracto urinario (sangre en la orina, micción dolorosa y frecuente, fiebre, dolor en la parte baja de la espalda)
- dolor en la parte superior del abdomen (dolor de estómago o molestias en la parte superior del estómago)
- fiebre (temperatura elevada)
- eczema (manchas rojas que pican en la piel)
- reacciones en el lugar de la inyección (dolor, enrojecimiento, hinchazón, picor y sensación de ardor en la piel cerca del lugar donde se puso la inyección)
- dolor de espalda
- artralgia (dolor articular)
- faringitis (dolor de garganta)
- congestión nasal (nariz taponada)
Poco frecuentes:pueden afectar hasta 1 de cada 100 personas
- herpes zóster (herpes)
Raros:pueden afectar hasta 1 de cada 1 000 personas
- reacciones alérgicas graves (anafilaxia)
- Si tiene alguno de estos síntomas, consulte a su médico o enfermero inmediatamente.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Nucala
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta o en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC)
No congelar.
Conservar en el embalaje original para protegerlo de la luz.
La jeringa precargada de Nucala se puede retirar de la nevera y conservar en el embalaje exterior sin abrir hasta un máximo de 7 días a temperatura ambiente (por debajo de 30 ºC), y protegida de la luz. Desechar si se deja fuera de la nevera más de 7 días.
6. Contenido del envase e información adicional
Composición de Nucala
El principio activo es mepolizumab.
Cada 1 ml de la jeringa precargada contiene 100 mg de mepolizumab.
Los demás componentes son: sacarosa, fosfato de sodio dibásico heptahidratado, ácido cítrico monohidratado, polisorbato 80, edetato de disodio, agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Nucala se presenta en una jeringa precargada de un solo uso como 1 ml de una solución de transparente a opalescente, de incolora a amarillo pálido-marrón pálido.
Nucala está disponible en un envase que contiene 1 jeringa precargada, o en un envase múltiple que contiene 3 x 1 jeringas precargadas o 9 x 1 jeringas precargadas.
Titular de la Autorización de Comercialización
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublín 24
Irlanda
Responsable de la fabricación
GlaxoSmithKline Manufacturing S.P.A
Strada Provinciale Asolana, No 90
43056 San Polo di Torrile, Parma
Italia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del Titular de la Autorización de Comercialización:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals s.a./n.v. Tél/Tel: + 32 (0) 10 85 52 00 | Lietuva UAB “BERLIN-CHEMIE MENARINI BALTIC” Tel: + 370 52 691 947 |
| Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals s.a./n.v. Belgique/Belgien Tél/Tel: + 32 (0) 10 85 52 00 |
Ceská republika GlaxoSmithKline, s.r.o. Tel: + 420 222 001 111 | Magyarország Berlin-Chemie/A. Menarini Kft. Tel.: + 36 23501301 |
Danmark GlaxoSmithKline Pharma A/S Tlf.: + 45 36 35 91 00 | Malta GlaxoSmithKline Trading Services Ltd. Tel: +356 80065004 |
Deutschland GlaxoSmithKline GmbH & Co. KG Tel.: + 49 (0)89 36044 8701 | Nederland GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Eesti OÜ Berlin-Chemie Menarini Eesti Tel: + 372 667 5001 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλ?δα GlaxoSmithKline Μονοπρ?σωπη A.E.B.E. Τηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
España GlaxoSmithKline S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel.: + 48 (0)22 576 9000 |
France Laboratoire GlaxoSmithKline Tél: + 33 (0)1 39 17 84 44 | Portugal GlaxoSmithKline – Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 |
Hrvatska Berlin-Chemie Menarini Hrvatska d.o.o. Tel: +385 1 4821 361 | România GlaxoSmithKline Trading Services Ltd. Tel: +40 800672524 |
Ireland GlaxoSmithKline (Ireland) Limited Tel: + 353 (0)1 4955000 | Slovenija Berlin-Chemie / A. Menarini Distribution Ljubljana d.o.o. Tel: + 386 (0)1 300 2160 |
Ísland Vistor hf. Sími: + 354 535 7000 | Slovenská republika Berlin-Chemie / A. Menarini Distribution Slovakia s.r.o. Tel: + 421 2 544 30 730 |
Italia GlaxoSmithKline S.p.A. Tel: + 39 (0)45 7741111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 (0)10 30 30 30 |
Κ?προς GlaxoSmithKline Trading Services Ltd. Τηλ: +357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija SIA Berlin-Chemie/Menarini Baltic Tel: + 371 67103210 | United Kingdom (Northern Ireland) GlaxoSmithKline Trading Services Ltd. Tel: + 44 (0)800 221441 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu
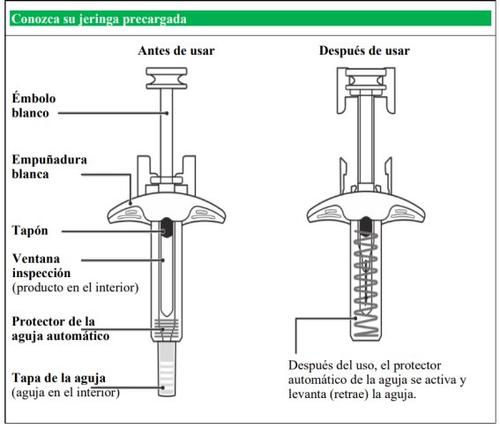
- Instrucciones de uso paso a paso de Nucala 100 mg jeringa precargada
Administración una vez cada 4 semanas.
Siga estas instrucciones acerca de cómo usar la jeringa precargada. El incumplimiento de estas instrucciones puede afectar al funcionamiento adecuado de la jeringa precargada. También debería recibir entrenamiento de cómo usar la jeringa precargada. Nucala jeringa precargada es solo para uso debajo de la piel(subcutáneo).
Cómo conservar Nucala
- Mantener refrigerado antes de su uso.
- No congelar.
- Conservar la jeringa precargada en el embalaje original para protegerlo de la luz.
- Mantener fuera de la vista y del alcance de los niños.
- Si es necesario, la jeringa precargada puede mantenerse a temperatura ambiente, por debajo de 30 ºC durante no más de 7 días, si se conserva en el embalaje original. Con cuidado, desechar la jeringa si se deja fuera de la nevera más de 7 días.
- Conservar por debajo de 30 ºC.
Antes de usar Nucala
La jeringa precargada debe utilizarse solo una vez y luego desecharse.
- Nocomparta su jeringa precargada con otra persona.
- Noagite la jeringa.
- Noutilice la jeringa si cae sobre una superficie dura.
- Noutilice la jeringa si parece estar dañada.
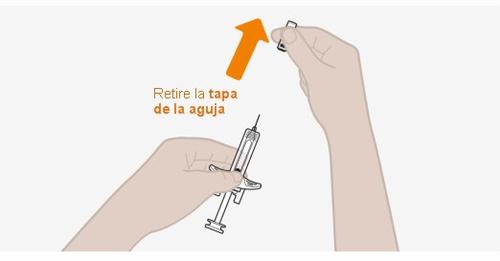
- Noretire la tapa de la aguja hasta justo antes de su inyección.
|
Prepare lo que necesite |
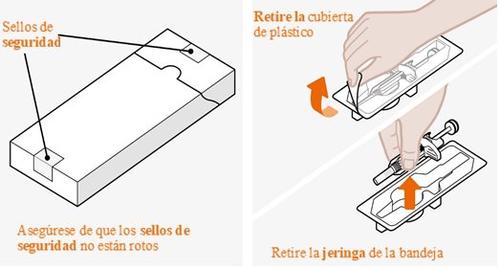
Encuentre una superficie cómoda, bien iluminada y limpia. Asegúrese de que tiene a su alcance:
|
|
|
Noutilice la jeringa si el sello de seguridad de la caja está roto. Noquite la tapa de la aguja en este momento. |
|
|
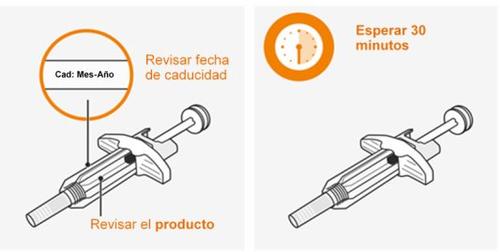
Noutilizar si la fecha de caducidad ha pasado. Nocaliente la jeringa en el microondas, con agua caliente o luz solar directa. Noinyecte la solución si se presenta turbia o descolorida, o tiene partículas. Noutilice la jeringa si se deja fuera del envase durante más de 8 horas. Noquite la tapa de la aguja en este momento. |
|
|
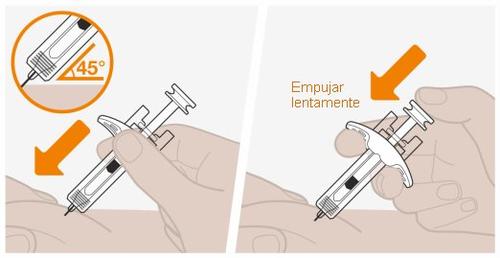
Noinyecte dónde su piel está magullada, sensible, enrojecida o dura. Noinyecte a una distancia inferior a los 5 cm de su ombligo. |
|
|
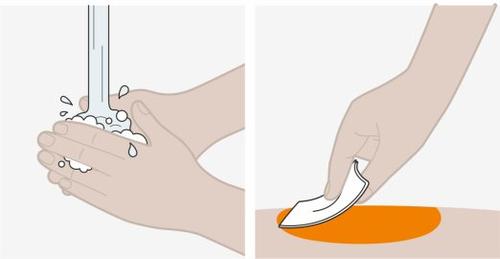
Novuelva a tocar el lugar de la inyección hasta que haya terminado. |
Inyectar |
|
|
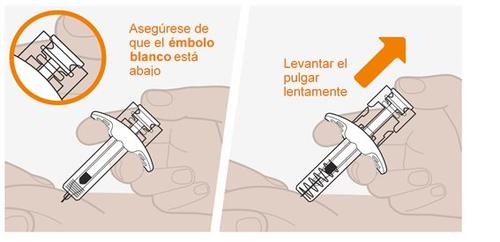
Nodeje que la aguja toque ninguna superficie. Notoque la aguja. Notoque el émbolo en esta etapa, ya que accidentalmente puede expulsar el líquido y no recibirá la dosis completa. Noexpulsar ninguna burbuja de aire de la jeringa. Novuelva a colocar la tapa de la aguja en la jeringa. Esto podría causar una lesión en la aguja. |
|
|
|
|
|
|
Desechar |
|
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NUCALA 100 MG SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 100 mgPrincipio activo: MepolizumabFabricante: Glaxosmithkline Trading Services LimitedRequiere recetaForma farmacéutica: INYECTABLE, 100 mgPrincipio activo: MepolizumabFabricante: Glaxosmithkline Trading Services LimitedRequiere recetaForma farmacéutica: INYECTABLE, 40 MGPrincipio activo: MepolizumabFabricante: Glaxosmithkline Trading Services LimitedRequiere receta
Médicos online para NUCALA 100 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NUCALA 100 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes