
NEUROMADE CÁPSULAS DURAS

Cómo usar NEUROMADE CÁPSULAS DURAS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
NEUROMADE cápsulas duras
Lea todo el prospecto detenidamente antes de empezar a tomar el medicamento.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Contenido del prospecto:
- Qué es Neuromade cápsulas y para qué se utiliza
- Antes de tomar Neuromade cápsulas
- Cómo tomar Neuromade cápsulas
- Posibles efectos adversos
- Conservación de Neuromade cápsulas
- Información adicional
1. Qué es NEUROMADE CÁPSULAS y para qué se utiliza
Neuromade cápsulas es una asociación de las vitaminas hidrosolubles del grupo B: tiamina (vitamina B1), piridoxina (vitamina B6) y cianocobalamina (vitamina B12) para su administración por vía oral.
Las vitaminas son nutrientes esenciales que intervienen en numerosos procesos del metabolismo humano.
Neuromade cápsulas está indicado en adultos y pacientes mayores de 14 años, en:
Tratamiento de estados de deficiencia de las vitaminas B1, B6 y B12, que podrían causar neuropatías (dolencias del sistema nervioso periférico) y podrían manifestarse en casos de dolor de espalda, como lumbalgias. Convalecencias. Dieta insuficiente.
2. Antes de tomar NEUROMADE CÁPSULAS
Notome NEUROMADE cápsulas
- si es alérgico (hipersensible) a los principios activos, a las cobalaminas (ej. hidroxobalamina), al cobalto o a cualquiera de los demás componentes del medicamento (ver la sección 6).
- si está en tratamiento con levodopa (medicamento para el Parkinson).
- si padece Enfermedad de Leber (atrofia del nervio óptico hereditaria) o ambliopía tabáquica (disminución de la agudeza visual, que se puede producir en personas que abusan del tabaco), porque podrían agravarse.
Por las dosis de vitaminas que contiene, no tome este medicamento:
- si está embarazada o en período de lactancia.
- niños menores de 14 años.
Tenga especial cuidado con NEUROMADE cápsulas
- No debe tomar una dosis más alta que la recomendada o durante un período de tiempo mayor que el recomendado. Cuando se administran de forma continuada grandes dosis de piridoxina (vitamina B6) se pueden producir efectos adversos de tipo neurológico (dolor de cabeza, sensación de hormigueo, etc.). Se han dado casos de dependencia y abstinencia al tomar durante un mes dosis de 200 mg de piridoxina.
- Por contener vitamina B12, si sufre alguna enfermedad de la sangre como alguna anemia, su médico deberá comprobar la causa antes de tomarla.
- Si tiene predisposición a padecer gota, por el contenido en vitamina B12 su médico deberá realizarle un especial control clínico porque podría recrudecerse.
- Si padece alguna enfermedad renal o del hígado no debe tomar este medicamento, pues las dosis que contiene exceden de las recomendadas en estas situaciones.
- Si padece afecciones tales como uremia (acumulación de urea en la sangre), infecciones, déficit de hierro o ácido fólico, o está en tratamiento con medicamentos que tienen un efecto supresor sobre la médula ósea (ej. cloranfenicol), la respuesta terapéutica a la vitamina B12 puede disminuir.
- Debe tener precaución con el sol o evitar exponerse a él, debido a que la piridoxina puede producir fotosensibilidad con aparición de erupción en la piel.
- Si hubiese padecido con anterioridad una alergia a la vitamina B1 al contacto con su piel (dermatitis de contacto) por motivos profesionales, podría sufrir una recaída al tomar este medicamento.
- Interferencias con pruebas analíticas: Si le van a realizar alguna prueba diagnóstica (incluidos análisis de sangre, orina, pruebas cutáneas que utilizan alergenos, etc...) comunique al médico que está tomando este medicamento, ya que puede alterar los resultados. En algunas determinaciones de urobilinógeno, teofilina y ácido úrico se podrían producir falsos resultados.
Uso de otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos incluso los adquiridos sin receta.
Neuromade cápsulas puede interaccionar con los siguientes medicamentos:
- Levodopa (medicamentos para el tratamiento del Parkinson).
- Fenobarbital, fenitoina, (medicamentos para la epilepsia).
- Amiodarona (para el corazón).
- Altretamina (para el tratamiento del cáncer).
- Medicamentos bloqueantes neuromusculares (utilizados en anestesia, para cirugía).
- Los siguientes medicamentos pueden interferir con la piridoxina (vitamina B6) y pueden reducir los niveles de esta vitamina, entre ellos: antibióticos para la tuberculosis (isoniazida, cicloserina, y etionamida), penicilamina (para enfermedades reumáticas), hidralazina (para la hipertensión), inmunosupresores como corticosteroides o ciclosporina y azatioprina (utilizados en el trasplante de órganos), ciclofosfamida (para el cáncer).
- Varios medicamentos pueden disminuir la absorción de cianocobalamina (vitamina B12) o reducir su efecto, como por ejemplo: ácido ascórbico (vitamina C) en grandes dosis, antibióticos aminoglucósidos, la colchicina (para el tratamiento de la gota), antagonistas H2 (medicamentos contra la acidez o úlcera de estómago, como ranitidina, cimetidina, etc.), ácido aminosalicílico (para enfermedades intestinales), omeprazol (para la úlcera de estómago), medicamentos para la epilepsia, metformina (para la diabetes), cloranfenicol (antibiótico), preparados de potasio de liberación sostenida o radiaciones de cobalto.
- Los anticonceptivos orales pueden reducir los niveles tanto de vitamina B6 como de vitamina B12.
Toma de NEUROMADE cápsulas con los alimentos y bebidas
Las bebidas alcohólicas disminuyen el efecto de las vitaminas.
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Por las dosis de vitaminas B que contiene este medicamento, mayores que las recomendadas durante el embarazo y la lactancia, está contraindicado durante estos periodos.
La tiamina, piridoxina y cianocobalamina pasan a la leche materna.
Conducción y uso de máquinas
Este medicamento puede producir somnolencia en unos pocos pacientes, los cuales no deberían conducir y /o utilizar máquinas durante el tratamiento.
3. Cómo tomar NEUROMADE CÁPSULAS
Siga exactamente las instrucciones de administración de Neuromade cápsulas indicadas por su médico.
Consulte a su médico o farmacéutico si tiene dudas.
La dosis normal es:
Se recomienda tomar 1 cápsula al día. En determinados casos el médico puede indicar la toma de 2 cápsulas al día.
Vía oral.
Las cápsulas se toman con la ayuda de una cantidad suficiente de agua.
En general, el tratamiento no debe superar 2 semanas, aunque el médico podría recomendar la administración durante más de 15 días.
Uso en niños
Este medicamento está contraindicado en niños menores de 14 años.
Si toma más NEUROMADE cápsulas del que debiera
No es de esperar que una ingestión de grandes dosis de Neuromade cápsulas produzca efectos tóxicos.
Si ha tomado más Neuromade cápsulas de lo que debe podría padecer síntomas como: molestias gastrointestinales (diarreas, náuseas y vómitos), trastornos nerviosos como reducción de la sensibilidad, hormigueos, adormecimiento en pies y manos; también podría aparecer dolor de cabeza, sensibilización a la luz del sol con lesiones en la piel; somnolencia, dificultad respiratoria, entre otros efectos, dependiendo de la dosis. En raras ocasiones, podría aparecer una reacción alérgica grave (shock anafiláctico).
En los niños, la ingestión accidental de dosis muy altas de vitamina B6 puede producir además sedación profunda, debilidad y dificultad respiratoria.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o acuda a un centro médico, o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar NEUROMADE cápsulas
No tome una dosis doble para compensar las dosis olvidadas
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Neuromade cápsulas puede producir efectos adversos, aunque no todas las personas los sufran.
La valoración de los efectos adversos que pueden producirse se basa en las siguientes frecuencias:
poco frecuentes (pueden afectar a entre 1 y 10 de cada 1.000 personas).
Con poca frecuencia pueden aparecer: náuseas, dolor de cabeza, efectos sobre la sensibilidad (parestesias) que se manifiestan como sensación de hormigueo en brazos y piernas o alteraciones en el tacto, somnolencia y erupción cutánea (reacciones de hipersensibilidad a las vitaminas B1, B6 y B12).
Otros efectos adversos que se han notificado, con frecuencia no conocida exactamente, son: molestias digestivas, diarrea, fotosensibilidad con lesiones en la piel como ampollas, mareo, inquietud; trastorno con reducción de la sensibilidad y hormigueos, andares inestables, adormecimiento de pies o manos, que generalmente disminuyen al interrumpirse el tratamiento; un síndrome de abstinencia a piridoxina más probable cuanto mayores son las dosis y en tratamientos superiores a un mes; cambios en el color de la orina, hinchazón e irritación en los ojos; ocasionalmente reacción anafiláctica con picor, hinchazón, dificultad respiratoria, etc.; algún caso de afección con nódulos y pus en la cara y el cuello.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de NEUROMADE CÁPSULAS
Mantener fuera del alcance y de la vista de los niños.
No utilice Neuromade cápsulas después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
No requiere condiciones especiales de conservación. Conservar en el embalaje original.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Información Adicional
Composición de NEUROMADE cápsulas duras
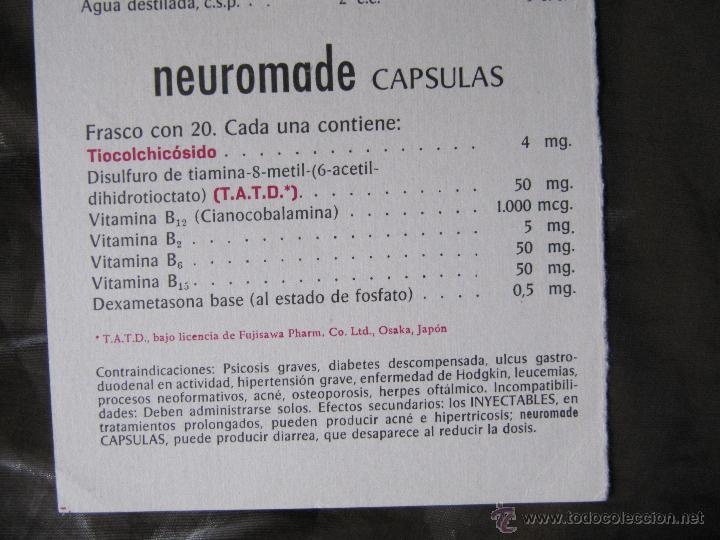
- Los principios activos son: Tiamina hidrocloruro (vitamina B1), Piridoxina hidrocloruro (vitamina B6) y Cianocobalamina (Vitamina B12). Cada cápsula contiene 50 mg de tiamina hidrocloruro, 50 mg de piridoxina hidrocloruro y 1.000 microgramos de cianocobalamina.
- Los demás componentes (excipientes) son: Celulosa microcristalina, anhídrido silícico y estearato magnésico
Aspecto del producto y contenido del envase
Neuromade cápsulas se presenta en envases de 20 cápsulas duras.
Titular de la autorización de comercialización y responsable de la fabricación
TEOFARMA Srl
Vía F.lli Cervi, 8
27010 Valle Salimbene (PV) – Italia
Este prospecto ha sido aprobado en Septiembre/2011.
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) ) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NEUROMADE CÁPSULAS DURASForma farmacéutica: COMPRIMIDO, 250/250/1000 mg/mg/mgPrincipio activo: Vitamin B1 in combination with vitamin B6 and/or vitamin B12Fabricante: Bayer Hispania S.L.No requiere recetaForma farmacéutica: COMPRIMIDO, 250 mg/250 mg/500 microgramosPrincipio activo: Vitamin B1 in combination with vitamin B6 and/or vitamin B12Fabricante: Almirall S.A.No requiere recetaForma farmacéutica: COMPRIMIDO, 250 mg / 250 mg /0,50 mgPrincipio activo: Vitamin B1 in combination with vitamin B6 and/or vitamin B12Fabricante: Laboratorios Normon S.A.No requiere receta
Médicos online para NEUROMADE CÁPSULAS DURAS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NEUROMADE CÁPSULAS DURAS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















