
NETDEX 3 MG/ML + 1 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS

Cómo usar NETDEX 3 MG/ML + 1 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
NETDEX 3mg/ml + 1mg/ml colirio en solución en envase unidosis
netilmicina/dexametasona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es NETDEX y para qué se utiliza
- Qué necesita saber antes de empezar a usar NETDEX
- Cómo usar NETDEX
- Posibles efectos adversos
- Conservación de NETDEX
- Contenido del envase e información adicional
1. Qué es NETDEX y para qué se utiliza
NETDEX contiene dos medicamentos: netilmicina y dexametasona.
- Netilmicina es un antibiótico que elimina las bacterias.
- Dexametasona es un corticosteroide que reduce la inflamación.
Los antibióticos se utilizan para tratar infecciones bacterianas y no sirven para tratar infecciones víricas. Es importante que siga las instrucciones relativas a la dosis, el intervalo de administración y la duración del tratamiento indicadas por su médico. No guarde ni reutilice este medicamento. Si una vez finalizado el tratamiento le sobra antibiótico, devuélvalo a la farmacia para su correcta eliminación. No debe tirar los medicamentos por el desagüe ni la basura. |
NETDEX se usa en adultos para reducir la inflamación y para eliminar bacterias en los ojos que están hinchados, irritados y con probabilidades de ser infectados por bacterias.
Debe consultar a un médico si empeora o si no mejora al final del tratamiento.
2. Qué necesita saber antes de empezar a usar NETDEX
Netilmicina/dexametasona puede usarse en adultos, incluidas las personas ancianas.
No se recomienda a personas de menos de 18 años de edad.
No useNETDEX:
- Si es alérgico a la netilmicina, la dexametasona, los antibióticos conocidos como antibióticos aminoglucósidos (como: tobramicina, kanamicina, amikacina, gentamicina, etc.) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si su médico le ha indicado que la presión del ojo es demasiado alta.
- Si cree que puede tener una infección vírica o fúngica en el ojo o alrededor del ojo.
- Si tiene ahora, o ha tenido en el pasado, una infección vírica del ojo causada por el virus del herpes simple (VHS).
- Si su médico le ha advertido de que tiene una infección del ojo causada por las bacterias conocidas como micobacterias.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar NETDEX.
Consulte a su médico si presenta hinchazón y aumento de peso alrededor del tronco y en la cara, ya que generalmente éstas son las primeras manifestaciones de un síndrome llamado síndrome de Cushing. Se puede producir supresión de la función de las glándulas suprarrenales tras interrumpir un tratamiento intensivo o a largo plazo con netilmicina/dexametasona. Consulte a su médico antes de interrumpir el tratamiento por su cuenta. Estos riesgos son especialmente importantes en niños y pacientes tratados con un medicamento llamado ritonavir o cobicistat.
Contacte con su médico si presenta visión borrosa u otros trastornos visuales.
Si usaNETDEXdurante un tiempo prolongado
- la presión del ojo puede aumentar y provocar daños a los nervios del ojo, causando problemas de visión. Si usa netilmicina/dexametasona durante más de 15 días, su médico debe comprobar su presión ocular de forma regular;
- puede desarrollar cataratas;
- las heridas pueden tardar más tiempo en curarse;
- es posible que su cuerpo no se defienda contra otros tipos de infecciones del ojo tan bien como sería lo normal, como las infecciones fúngicas o víricas;
- las infecciones oculares que producen mucho pus, con el uso de corticosteroides pueden empeorar o puede que sea más difícil identificar el tipo de bacterias que causan la infección;
- el corticosteroide que contiene netilmicina/dexametasona puede provocar el estrechamiento de las superficies del ojo e incluso perforaciones;
- puede empezar a ser alérgico al antibiótico que contiene el colirio.
Antes de usar NETDEX informe a su médico si
- tiene glaucoma o antecedentes familiares de glaucoma;
- tiene problemas de córnea;
- está tomando otros medicamentos que contienen fosfatos. Su médico hará un seguimiento de su córnea a intervalos regulares.
Niños y adolescentes
NETDEX no se recomienda para su uso en niños o adolescentes (desde el nacimiento hasta los 18 años de edad).
Solo para uso externo
Use solo NETDEX en la superficie del ojo. Este medicamento no debe inyectarse ni tragarse.
Uso de NETDEX con otros medicamentos
NETDEX puede interactuar con otros medicamentos. Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar algún producto de uso oftálmico o cualquier otro medicamento, incluidos los medicamentos obtenidos sin receta. Puede usar igualmente netilmicina/dexametasona con otros productos oftálmicos, pero debe seguir las instrucciones de la sección 3.
Especialmente, informe a su médico o farmacéutico si está tomando:
- cualquier otro antibiótico, en particular polimixina B, colistina, viomicina, estreptomicina, vancomicina o cefaloridina. El uso simultáneo de netilmicina/dexametasona con otros antibióticos podría aumentar el riesgo de problemas de riñón, problemas de oído, o podría afectar al buen funcionamiento de los otros antibióticos;
- cisplatino (un medicamento anticancerígeno);
- diuréticos (medicinas que reducen la retención de líquidos) como ácido etacrínico y furosemida;
- medicamentos anticolinérgicos (medicamentos que detienen la secreción de las glándulas), como la atropina;
- ritonavir o cobicistat, ya que éstos pueden aumentar la cantidad de dexametasona en la sangre;
- otros medicamentos que contengan fosfatos. Su médico hará un seguimiento de su córnea a intervalos regulares.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Uso durante el embarazo
Es preferible no usar NETDEX durante el embarazo a menos que su médico lo considere necesario.
Uso durante la lactancia
NETDEX no debe utilizarse si está en periodo de lactancia.
Conducción y uso de máquinas
Cuando usa NETDEX su visión puede volverse borrosa durante un corto periodo de tiempo. Si esto sucede, no conduzca ni use máquinas hasta que su visión recupere la claridad.
NETDEX contiene fosfatos
Este medicamento contiene 0,18 mg de fosfatos en cada gota equivalente a 3,66 mg/ml. Si sufre de daño grave en la córnea (la capa trasparente de la parte frontal del ojo) el tratamiento con fosfatos, en casos muy raros, puede provocar visión borrosa por acumulación de calcio.
3. Cómo usar NETDEX
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es una gotaen el ojo afectado cuatro veces al díao según se le haya prescrito. La duración normal del tratamiento puede variar de 5 a 14 días.
No cambie la dosis del colirio sin consultar a su médico.
Uso en niños y adolescentes
NETDEX no se recomienda para su uso en niños o adolescentes (desde el nacimiento hasta los 18 años de edad).
Usuarios de lentes de contacto
NETDEX en envases unidosis puede usarse mientras usa lentes de contacto porque no contiene conservantes. No obstante, se recomienda encarecidamente evitar llevar lentes de contacto durante una infección o inflamación ocular. No se deben usar lentes de contacto durante el tratamiento con colirios con corticosteroides, debido al riesgo incrementado de infección.
Si usa NETDEX con otros colirios oftálmicos
Espere al menos 10 minutos entre el uso de NETDEX y el uso de otros colirios o pomadas oculares. Las pomadas oftálmicas deben utilizarse en último lugar.
Instrucciones de uso
Asegúrese de que el envase unidosis está intacto.
- Lávese las manos y siéntese o colóquese de forma cómoda.
- Abra el sobre de aluminio que contiene los envases unidosis.
- Desprenda un envase unidosis de la tira (Dibujo 1) y vuelva a meter los envases sin abrir en el sobre.
- Abra girando la parte superior sin tirar (Dibujo 2). No toque la punta tras abrir el envase.
- Incline la cabeza hacia atrás.
- Con el dedo, retire ligeramente el párpado inferior del ojo afectado.
- Invierta el envase unidosis y coloque la punta del envase cerca del ojo, pero sin tocarlo. No toque el ojo o el párpado con la punta del envase (Dibujo 3).
- Presione el envase unidosis para administrar solo una gota, y luego suelte el párpado inferior.
- Cierre el ojo y presione con un dedo sobre la esquina del ojo afectado, al lado de la nariz. Sujete durante 2 minutos.
- Repita en el otro ojo si su médico se lo ha indicado.
- Deseche el envase unidosis tras el uso.

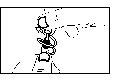
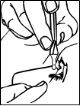
Si usa el colirio de forma incorrecta, éste puede sufrir contaminación por bacterias, lo que puede llevar a infecciones oculares. El uso de colirio contaminado puede causar daño ocular grave y posterior pérdida de visión.
Si usa más NETDEX del que debe
Si usa demasiado colirio no es probable que tenga problemas.
Aplique la siguiente dosis como de costumbre.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico, farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad tomada.
Si olvidó usar NETDEX
No use una dosis doble para compensar las dosis olvidadas.
Aplique la siguiente dosis como de costumbre.
Si interrumpe el tratamiento con NETDEX
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
La frecuencia del efecto en cada persona no se puede estimar a partir de los datos disponibles.
Trastornos oculares
Aumento de la presión ocular, formación de cataratas tras tratamientos prolongados, visión borrosa, desarrollo o empeoramiento de una infección vírica del ojo causada por el virus del herpes simple (VHS) o una infección fúngica, retraso en la cicatrización de heridas.
En casos muy raros (menos de 1 de cada 10.000 personas), algunos pacientes con daño grave en la capa transparente de delante del ojo (la córnea) han desarrollado manchas opacas en la córnea debido a la acumulación de calcio durante el tratamiento.
Trastornos del sistema inmunológico
Reacción alérgica local: enrojecimiento de la conjuntiva, quemazón, picor.
Trastornos hormonales
Crecimiento de más vello corporal (particularmente en las mujeres), debilidad y desgaste muscular, estrías moradas en la piel del cuerpo, aumento de la presión arterial, menstruaciones irregulares o ausentes, cambios en los niveles de proteínas y calcio del cuerpo, retraso en el crecimiento en niños y adolescentes e hinchazón y aumento de peso del cuerpo y la cara (llamado síndrome de ‘Cushing’) (ver sección 2 “Advertencias y precauciones”).
En todos los casos mencionados anteriormente, se recomienda interrumpir el tratamiento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de NETDEX
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja, el sobre y el envase después de "CAD". La fecha de caducidad es el último día del mes que se indica.
Conservar por debajo de 30ºC.
Conservar los envases unidosis en el sobre para protegerlos de la luz y la humedad.
Este medicamento no contiene conservantes.
Tras la primera apertura de un envase unidosis, úselo inmediatamente y deseche el envase unidosis con todo el contenido restante tras el uso.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de NETDEX
Los principios activos son dexametasona 1 mg/ml (en forma de fosfato disódico de dexametasona) y netilmicina 3 mg/ml (como sulfato de netilmicina). Cada envase unidosis contiene 0,9 mg de netilmicina y 0,3 mg de dexametasona.
Los demás componentes son:
citrato de sodio,
fosfato de sodio monobásico monohidrato,
fosfato disódico dodecahidrato,
agua purificada.
Aspecto del producto y contenido del envase
NETDEX es una solución transparente, incolora o ligeramente amarillenta.
NETDEX colirio en envases unidosis:
Cinco envases unidosis, cada uno con 0,3 ml de NETDEX colirio en solución, envueltos en un sobre de aluminio.
Cada envase contiene 15 o 20 envases unidosis.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
SIFI S.p.A.
Via Ercole Patti 36
95025 Aci Sant'Antonio (CT)
Italia
Representante local:
SIFI IBÉRICA S.L.
C/ Poeta Joan Maragall, 47
Planta 4, Puerta 402
28020 Madrid - España
Fecha de la última revisión de este prospecto:Enero 2020
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.es.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NETDEX 3 MG/ML + 1 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSISForma farmacéutica: COLIRIO, 1 mg/ml + 5 mg/mlPrincipio activo: dexamethasone and antiinfectivesFabricante: Santen OyRequiere recetaForma farmacéutica: COLIRIO, -Principio activo: dexamethasone and antiinfectivesFabricante: Novartis Farmaceutica S.A.Requiere recetaForma farmacéutica: COLIRIO, 3mg/ml +1 mg/mlPrincipio activo: dexamethasone and antiinfectivesFabricante: Sifi S.P.A.Requiere receta
Médicos online para NETDEX 3 MG/ML + 1 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NETDEX 3 MG/ML + 1 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















