
NEBIVOLOL TEVA-RATIOPHARM 5 mg COMPRIMIDOS EFG

Cómo usar NEBIVOLOL TEVA-RATIOPHARM 5 mg COMPRIMIDOS EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Nebivolol Teva-ratiopharm 5 mg comprimidos EFG
nebivolol
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4..
Contenido del prospecto:
- Qué es Nebivolol Teva-ratiopharm y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Nebivolol Teva-ratiopharm
- Cómo tomar Nebivolol Teva-ratiopharm
- Posibles efectos adversos
- Conservación de Nebivolol Teva-ratiopharm
- Contenido del envase e información adicional
1. Qué es Nebivolol Teva-ratiopharm y para qué se utiliza
Nebivolol Teva-ratiopharm contiene nebivolol, un medicamento con acción cardiovascular, perteneciente al grupo de agentes beta-bloqueantes selectivos (esto es, con actividad selectiva en el sistema cardiovascular). Previene el aumento de la frecuencia cardiaca, y controla la fuerza de bombeo del corazón. Ejerce también una acción dilatadora de los vasos sanguíneos, lo cual contribuye a su vez a disminuir la presión arterial.
Nebivolol Teva-ratiopharm se utiliza en el tratamiento de pacientes con:
- Tensión arterial elevada (hipertensión)
- Insuficiencia cardiaca crónica leve y moderada en pacientes de 70 años o más, en adición a otros tratamientos.
2. Qué necesita saber antes de empezar a tomar Nebivolol Teva-ratiopharm
No tome Nebivolol Teva-ratiopharm
- Si es alérgico al nebivolol o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si tiene problemas de hígado.
- Si tiene insuficiencia cardiaca, que se acaba de producir o un empeoramiento reciente de la misma, o está recibiendo tratamiento intravenoso para ayudar a trabajar el corazón, después de sufrir un colapso circulatorio debido a una insuficiencia cardiaca aguda.
- Si sufre trastornos del ritmo del corazón (tales como síndrome del seno enfermo incluyendo bloqueo sinoatrial).
- Si sufre trastornos en la conducción cardiaca (como bloqueo cardiaco de segundo o tercer grado y no utiliza marcapasos).
- Si tiene asma o ha sufrido alguna vez dificultad en la respiración o respiración sibilante.
- Si tiene la presión arterial elevada, sofocos o diarrea producido por un tumor no tratado de la médula adrenal (feocromocitoma).
- Si tiene un trastorno metabólico donde hay un cambio en el equilibrio ácido/base del cuerpo (acidosis metabólica).
- Si tiene el ritmo cardiaco lento (menos de 60 latidos por minuto antes del inicio del tratamiento con este medicamento).
- Si tiene la tensión arterial baja (tensión arterial sistólica menos de 90 mmHg).
- Si tiene circulación sanguínea deficiente en brazos o piernas.
Advertencias y precauciones
Consulte a su médico antes de empezar a tomar Nebivolol Teva-ratiopharm.
Informe a su médico si usted padece alguno de las siguientes condiciones:
- Si se va a someter a una operación que requiere un anestésico, informe siempre a su anestesista que está tomando Nebivolol Teva-ratiopharm antes de ser anestesiado
- Insuficiencia cardiaca crónica sin tratamiento
- Latido del corazón anormalmente lento
- Si padece problemas de circulación en los brazos o piernas (tales como enfermedad o síndrome de Raynaud, claudicación intermitente), dolor al caminar parecido a un calambre
- Si padece bloqueo cardiaco de primer grado.
- Si padece dolor en el pecho en reposo que se produce en ciclos (angina de Prinzmetal).
- Si usted es diabético, ya que nebivolol puede enmascarar los síntomas producidos por azúcar bajo en sangre (hipoglucemia) y podría aumentar el riesgo de hipoglucemia severa cuando se utiliza con cierto tipo de medicamentos antidiabéticos llamados sulfonilureas (por ejemplo, gliquidona, gliclazida, glibenclamida, glipizida, glimepirida o tolbutamida).
- Si sufre problemas de tiroides, ya que nebivolol puede enmascarar los síntomas de frecuencia cardiaca elevada (taquicardia)
- Si presenta zonas engrosadas e irritadas de la piel (psoriasis).
- Si padece reacciones alérgicas, ya que nebivolol puede aumentar la gravedad de esas reacciones.
- En pacientes con trastornos pulmonares obstructivos crónicos, los antagonistas beta-adrenérgicos deberán utilizarse con precaución ya que puede agravarse la constricción de las vías aéreas.
- Si padece problemas de respiración prolongados.
Si usted padece alguna alteración renal grave, consulte a su médico antes de tomar Nebivolol Teva-ratiopharm para tratar su insuficiencia cardiaca.
Al inicio del tratamiento de una insuficiencia cardiaca crónica, deberá ser regularmente monitorizado por un médico experimentado (ver sección 3). Este tratamiento no se debería suspender bruscamente, a menos que sea claramente indicado y evaluado por su médico (ver sección 3).
Niños y adolescentes
Nose recomienda el uso de Nebivolol Teva-ratiopharm en niños y adolescentes debido a la ausencia de datos sobre el uso de este medicamento en este tipo de pacientes.
Toma de Nebivolol Teva-ratiopharm con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
Informe a su médico si está tomando cualquiera de lo siguiente:
- Algunos medicamentos para el corazón o para el control de la presión arterial (tales como amiodarona, amlodipino, cibenzolina, clonidina, digoxina, diltiazem, disopiramida, felodipino, flecainida, guanfacina, hidroquinidina, lacidipino, lidocaína, metildopa, mexiletina, moxonidina, nicardipino, nifedipino, nimodipino, nitrendipino, propafenona, quinidina, rilmenidina, verapamilo).
- Sedantes y medicamentos para la enfermedad mental (psicosis), como barbitúricos (también usados para la epilepsia), fenotiazinas (también usados para vómitos y náuseas) y tioridazina.
- Medicamentos para la depresión, como amitriptilina, paroxetina y fluoxetina.
- Medicamentos usados para la anestesia durante una operación.
- Medicamentos para el asma, descongestionantes nasales y algunos medicamentos para tratar alteraciones oculares como el glaucoma (incremento de la presión del ojo) o dilatación de la pupila.
- Baclofeno (un medicamento antiespasmódico); Amifostina (un medicamento protector utilizado durante el tratamiento del cáncer).
- Medicamentos para la diabetes, como insulina o antidiabéticos orales
Todos estos medicamentos, al igual que Nebivolol Teva-ratiopharm pueden influir en la presión arterial y en la función del corazón.
Medicamentos para tratar un exceso de acidez en el estómago o úlceras (medicamentos antiácidos): debe tomar Nebivolol Teva-ratiopharm durante la comida, y el antiácido entre comidas.
Toma de Nebivolol Teva-ratiopharm con alimentos y bebidas
Ver sección 3.
Embarazo y lactancia
Nebivolol Teva-ratiopharm no debe administrarse durante el embarazo a menos que su médico lo considere necesario. No se recimienda su uso durante la lactancia.
Si está embarazada o en periodo de lactancia, cree que podría está embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Nebivolol Teva-ratiopharm puede producir mareo y fatiga. En estos casos, no conduzca ni utilice maquinaria.
Nebivolol Teva-ratiopharm contiene lactosa
Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Nebivolol Teva-ratiopharm contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por comprimido; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Nebivolol Teva-ratiopharm
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Forma de administración:
El comprimido se debe ingerir con una cantidad suficiente de líquido (por ej. un vaso de agua). El comprimido se puede tomar con o sin alimento. El comprimido se puede dividir en mitades y cuartos iguales.
Si su médico le ha indicado que tome ¼ (cuarto) o ½ (medio) comprimido diario, por favor siga las siguientes instrucciones para fraccionar los comprimidos de 5 mg de nebivolol, ranurados en forma de cruz.
- Colocar los comprimidos sobre una superficie plana, dura (ej. mesa o encimera), con la ranura en forma de cruz cara arriba.
- Romper el comprimido presionándolo con los dedos índices de ambas manos colocados al lado de una de las ranuras (dibujos 1 y 2).
- Proceder del mismo modo para fraccionar la mitad del comprimido en un cuarto (dibujos 3 y 4).
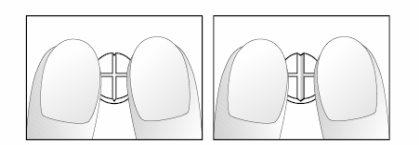
Dibujos 1 y 2: fácil rotura del comprimido de 5 mg de nebivolol ranurado en forma de cruz en una mitad.
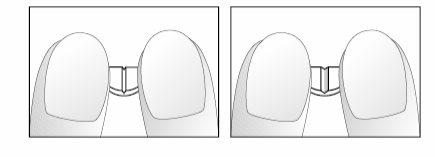
Dibujos 3 y 4: Fácil rotura de la mitad del comprimido de 5 mg de nebivolol ranurado en forma de cruz en un cuarto.
Presión arterial elevada (hipertensión)
Adultos
La dosis recomendada es 5 mg (un comprimido) al día, preferentemente a la misma hora del día.
El efecto hipotensor se alcanza después de 1-2 semanas de tratamiento. Ocasionalmente, se alcanza el efecto óptimo solo después de 4 semanas.
Pacientes con problemas de riñón
Si tiene problemas de riñón, la dosis inicial recomendada es 2,5 mg al día (medio comprimido). Si es necesario, su médico le aumentará la dosis diaria hasta 5 mg.
Personas de edad avanzada
Si usted es mayor de 65 años, la dosis inicial recomendada es 2,5 mg al día (medio comprimido). Si es necesario, su médico aumentará su dosis diaria a 5 mg.
Uso en niños y adolescentes
Nebivolol no está recomendado para su uso en niños y adolescentes menores de 18 años.
Insuficiencia cardiaca crónica
Adultos
- Su tratamiento debe iniciarse siempre bajo control médico.
- Su médico empezará su tratamiento con ¼ (cuarto) de comprimido al día. La dosis se incrementará después de 1-2 semanas hasta ½ (medio) comprimido al día, después hasta 1 comprimido al día y luego hasta 2 comprimidos al día hasta conseguir la dosis óptima para usted. Su médico le prescribirá la dosis correcta para usted en cada momento y usted deberá seguir exactamente sus instrucciones.
- La dosis máxima recomendada es de 2 comprimidos (10 mg) al día.
- El inicio del tratamiento y cada aumento de dosis se le realizará bajo la supervisión de un médico experimentado durante un periodo de 2 horas.
- Su médico le reducirá su dosis en caso que sea necesario.
- No deberá interrumpir bruscamente el tratamiento, ya que esto podría empeorar su insuficiencia cardiaca.
- Pacientes con problemas graves de riñón, no deberán tomar este medicamento.
- Tome el medicamento una vez al día, preferiblemente a la misma hora del día.
Uso en niños y adolescentes
Nebivolol no está recomendado para su uso en niños y adolescentes menores de 18 años.
Combinación con otros medicamentos
Su médico puede decidir combinar Nebivolol Teva-ratiopharm con otros medicamentos para tratar su enfermedad.
Si toma más Nebivolol Teva-ratiopharm del que debiera
Los síntomas y signos más frecuentes de sobredosis de nebivolol son ritmo cardiaco muy lento (bradicardia), presión arterial baja con posibilidad de desmayo (hipotensión), dificultad en la respiración como en el asma (broncoespasmo) e insuficiencia cardiaca aguda.
En caso de sobredosis o ingestión accidental, consulte inmediatamente con su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20 indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Nebivolol Teva-ratiopharm
Si ha olvidado una dosis de nebivolol, pero lo recuerda en un corto plazo, tome el comprimido siguiente normalmente. Sin embargo, si se produce un retraso largo (es decir, varias horas), de manera que la dosis siguiente está cerca, sáltese la dosis olvidada y tome la siguiente dosis normal, programada en la hora habitual. No tome una dosis doble. Sin embargo, se debe evitar el olvido repetido de la toma del medicamento.
Si interrumpe el tratamiento con Nebivolol Teva-ratiopharm
Consulte siempre a su médico antes de interrumpir el tratamiento con Nebivolol Teva-ratiopharm, tanto si usted lo toma para la presión arterial elevada como para la insuficiencia cardiaca crónica. No debe interrumpir bruscamente el tratamiento, ya que esto podría empeorar temporalmente su insuficiencia cardiaca. Si fuera necesario interrumpir el tratamiento para la insuficiencia cardiaca crónica, la dosis diaria debe disminuirse gradualmente, partiendo la dosis por la mitad, a intervalos de una semana.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Cuando se utilizaNebivolol Teva-ratiopharm para el tratamiento de la presión arterial elevada, los posibles efectos adversos son:
Frecuentes (puede afectar hasta 1 de cada 10 personas):
- dolor de cabeza
- mareo
- cansancio
- picor inusual o sensación de hormigueo
- diarrea
- estreñimiento
- náuseas
- dificultad para respirar
- manos o pies hinchados.
Poco frecuentes (puede afectar hasta 1 de cada 100 personas):
- frecuencia cardiaca lenta u otras dolencias cardiacas
- presión arterial baja
- dolor al caminar parecido a un calambre
- visión anormal
- impotencia (disfunción eréctil)
- dificultad para respirar como en el asma, debido a contracción repentina de los músculos de alrededor de las vías respiratorias (espasmo bronquial)
- indigestión, gases en el estómago o intestino, vómitos
- picor, erupción de la piel
- pesadillas
- sentimiento de depresión
Muy raros (puede afectar hasta 1 de cada 10.000 personas):
- desmayos.
- empeoramiento de la psoriasis (erupción cutánea).
La frecuencia de los siguientes efectos adversos es no conocida:
- reacciones alérgicas, tales como erupciones cutáneas generalizadas (reacciones de hipersensibilidad).
- hinchazón súbita de la zona de los labios, párpados o de la lengua pudiendo acompañarse de dificultad respiratoria aguda (angioedema)
- erupción en la piel caracterizada por ronchas rosadas, con relieve, que producen picor, de causa alérgica o no alérgica (urticaria).
En un estudio clínico para la insuficiencia cardiaca crónica, se vieron los siguientes efectos adversos:
Efectos adversos muy frecuentes (puede afectar a más de 1 de cada 10 personas):
- latido del corazón lento
- mareo
Efectos adversos frecuentes (puede afectar hasta 1 de cada 10 personas):
- agravamiento de la insuficiencia cardiaca
- presión arterial baja (como sensación de desvanecimiento al levantarse rápidamente)
- intolerancia a este medicamento
- alteración leve de la conducción cardiaca afectando al ritmo cardiaco (bloqueo auriculo-ventricular de 1er grado)
- hinchazón de las extremidades inferiores (aumento volumen tobillos).
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento
5. Conservación de Nebivolol Teva-ratiopharm
Mantener fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el blister y en el envase. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Nebivolol Teva-ratiopharm
El principio activo es nebivolol (como hidrocloruro). Cada comprimido contiene 5 mg de nebivolol equivalente a 5,45 mg de nebivolol hidrocloruro.
Los demás componentes son: lactosa monohidrato, croscarmelosa sódica, sílice coloidal anhidra, macrogol 6000 y estearato magnésico.
Aspecto del producto y contenido del envase
Comprimido redondo, blanco, biconvexo con un diámetro de 9 mm, con ranura en forma de cruz en una cara y marcado con “N 5” en la otra cara.
Los comprimidos se proporcionan en blister de PVC/PE/PVdC//Al o blister unidosis PVC/PE/PVdC//Al transparente e incoloro. Tamaños de envase: 7, 8, 10, 14, 15, 20, 28,30, 50, 56, 60, 90, 98, 100, 500 y 50 x 1 blister unidosis (envase hospitalario).
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Teva Pharma S.L.U.
C/ Anabel Segura nº 11, Edificio Albatros B, 1ª planta
Alcobendas 28108 Madrid
Responsable de la fabricación
Balkanpharma Dupnitsa AD,
3 Samokovsko Shosse Str.,
Dupnitsa 2600,
Bulgaria
ó
Actavis Ltd,
BLB 015-016, Bulebel Industrial Estate,
Zejtun ZTN 3000,
Malta
Este medicamento está autorizado en los Estados Miembros del Espacio Económico Europeo con los siguientes nombres:
Bélgica: Nebivolol Teva 5 mg tabletten
Bulgaria: Nebivolol Teva 5 mg tablets
España: Nebivolol Teva-ratiopharm 5 mg comprimidos EFG
Estonia: NEBIPHAR
Hungría: Nebivolol-Teva 5 mg tabletta
Irlanda: Nebivolol Teva 5 mg Tablets
Italia: Nebivololo Teva Italia 5 mg compresse
Países Bajos:Nebivolol Teva 5mg, tabletten
Fecha de la última revisión de este prospecto:Junio 2021
Otras fuentes de información
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
Puede acceder a información detallada y actualizada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el cartonaje. También puede acceder a esta información en la siguiente dirección de internet: https://cima.aemps.es/cima/dochtml/p/72140/P_72140.html
Código QR + URL
- País de registro
- Precio medio en farmacia7.87 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NEBIVOLOL TEVA-RATIOPHARM 5 mg COMPRIMIDOS EFGForma farmacéutica: COMPRIMIDO, 10 mgPrincipio activo: nebivololFabricante: Glenmark Arzneimittel GmbhRequiere recetaForma farmacéutica: COMPRIMIDO, 2.5 MGPrincipio activo: nebivololFabricante: Glenmark Arzneimittel GmbhRequiere recetaForma farmacéutica: COMPRIMIDO, 5 MGPrincipio activo: nebivololFabricante: Glenmark Arzneimittel GmbhRequiere receta
Médicos online para NEBIVOLOL TEVA-RATIOPHARM 5 mg COMPRIMIDOS EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NEBIVOLOL TEVA-RATIOPHARM 5 mg COMPRIMIDOS EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











