
MOMETASONA FUROATO TEVA 50 MICROGRAMOS SUSPENSION PARA PULVERIZACION NASAL


Cómo usar MOMETASONA FUROATO TEVA 50 MICROGRAMOS SUSPENSION PARA PULVERIZACION NASAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para elusuario
MometasonafuroatoTeva 50 microgramos suspensión para pulverización nasal
furoato de mometasona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted,y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Mometasona furoato Teva y para qué se utiliza
- Qué necesita saber antes de empezar a usar Mometasona furoato Teva
- Cómo usar Mometasona furoato Teva
- Posibles efectos adversos
- Conservación de Mometasona furoato Teva
- Contenido del envase e información adicional
1. Qué es Mometasona furoato Teva y para qué se utiliza
¿Qué es MometasonafuroatoTeva?
Mometasona furoato Teva pulverizador nasal contiene furoato de mometasona, un medicamento de un grupo de medicamentos llamados corticoides. Furoato de mometasona no debe confundirse con los esteroides “anabolizantes” usados de forma indebida por algunos atletas y administrados en comprimidos o en inyección. Cuando se pulveriza furoato de mometasona en la nariz, puede ayudar a aliviar la inflamación (hinchazón e irritación de la nariz), los estornudos, el picor y el taponamiento o el goteo nasal.
¿Para qué se utiliza MometasonafuroatoTeva?
Fiebre del heno y rinitis perenne
Mometasona furoato Teva se utiliza para tratar los síntomas de la fiebre del heno (también llamada rinitis alérgica estacional) y rinitis perenne en adultos y niños de 3 años de edad y mayores.
La fiebre del heno, que se presenta en ciertas épocas del año, es una reacción alérgica causada por respirar polen de árboles, hierbas, malezas y también esporas de mohos y hongos. La rinitis perenne se presenta durante todo el año y los síntomas pueden ser causados por una sensibilidad a una variedad de cosas incluyendo los ácaros del polvo, pelo del animal (o epitelio), plumas y ciertos alimentos. Mometasona furoato Teva reduce la inflamación e irritación de su nariz y también alivia los estornudos, el picor y el taponamiento o el goteo nasal causados por la fiebre del heno o la rinitis perenne.
Pólipos nasales
Mometasona furoato Teva se utiliza para tratar los pólipos nasales en adultos de 18 años y mayores.
Los pólipos nasales son unas pequeñas formaciones en la mucosa de la nariz y generalmente afectan a ambas fosas nasales. Mometasona furoato Teva reduce la inflamación en la nariz, haciendo que los pólipos se reduzcan gradualmente, de este modo alivia el taponamiento nasal que puede afectar a su respiración por la nariz.
2. Qué necesita saber antes de empezar a tomar Mometasona furoato Teva
No use Mometasona furoatoTeva:
- si es alérgico a furoato de mometasona o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si presenta infección nasal no tratada. El uso de Mometasona furoato Teva mientras tiene una infección de nariz no tratada, como el herpes, puede empeorar la infección. Debe esperar hasta que la infección desaparezca antes de comenzar a utilizar el pulverizador nasal.
- si ha sido sometido recientemente a una intervención quirúrgica de la nariz o ha sufrido una lesión reciente en la nariz. No debe utilizar el pulverizador nasal hasta que haya cicatrizado la nariz.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Mometasona furoato Teva.
- si tiene o ha tenido alguna vez tuberculosis
- si tiene cualquier otra infección
- si está tomando otros corticosteroides, por vía oral o mediante inyección
- si tiene fibrosis quística
Mientras está usando Mometasona furoato Teva, consulte a su médico:
- si su sistema inmunológico no está funcionando bien (si tiene dificultad en superar la infección) y entra en contacto con alguien con sarampión o varicela. Debe evitar el contacto con cualquier persona que tenga estas infecciones.
- si tiene infección de nariz o garganta.
- si lleva usando este medicamento varios meses o más tiempo.
- si tiene irritación persistente de nariz o garganta.
Si los pulverizadores nasales de corticosteroides se usan a dosis altas durante largos periodos de tiempo, se pueden producir efectos adversos, debido a que el medicamento se absorbe en el organismo.
Si le pican los ojos o están irritados, su médico puede aconsejarle el uso de otros tratamientos a la vez que Mometasona furoato Teva.
Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Niños
Cuando se usan a dosis altas durante largos periodos de tiempo, los pulverizadores nasales de corticosteroides pueden causar efectos secundarios, tales como enlentecimiento del índice de crecimiento en los niños.
Se recomienda controlar la altura de los niños en tratamiento de larga duración con corticosteroides nasales y si se aprecia cualquier cambio, debe comunicárselo al médico.
Uso de Mometasona furoatoTeva con otros medicamentos
Comunique a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento, incluidos los adquiridos sin receta.
Si está tomando otros corticosteroides para la alergia, por vía oral o mediante inyección, su médico puede aconsejarle que deje de tomarlos cuando empiece a usar Mometasona Teva. Al discontinuar los corticosteroides por vía oral o en inyección algunas personas pueden presentar algunos efectos adversos, como dolor muscular o de las articulaciones, debilidad y depresión. También puede que desarrolle otras alergias, tales como ojos llorosos y con picor, o piel con ronchas rojas. Si desarrolla alguno de estos efectos debe consultar a su médico.
Algunos medicamentos pueden incremetar los efectos de mometasona furoato, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos medicamentos para tratar el VIH como ritonavir, cobicistat).
Embarazo, lactancia y fertilidad
Hay poca información o ninguna, sobre el uso de Mometasona furoato Teva en mujeres embarazadas. Se desconoce si furoato de mometasona se excreta en la leche materna.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
No existe ninguna información conocida sobre el efecto de Mometasona furoato Teva en la conducción o uso de máquinas.
Mometasona furoato Tevacontiene cloruro de benzalconio
Este medicamento contiene 20 microgramos de cloruro de benzalconio en cada pulverización (0,1 ml). El cloruro de benzalconio puede causar irritación o inflamación dentro de la nariz, especialmente cuando se usa durante un tratamiento a largo plazo.
3. Cómo tomar Mometasona Teva
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico. No use dosis mayores ni utilice el pulverizador más a menudo o durante más tiempo de lo que su médico le ha dicho.
Tratamiento de la fiebre del heno y rinitis perenne
Uso en adultos y niños de más de 12 años de edad
La dosis recomendada es de dos pulverizaciones en cada orificio nasal una vez al día
- Una vez que se ha alcanzado el control de los síntomas, es posible que su médico le recomiende disminuir la dosis a una pulverización en cada orificio nasal una vez al día.
- Si no comienza a sentirse mejor, debe consultar al médico y puede que aumente la dosis a la dosis máxima diaria de cuatro pulverizaciones en cada orificio nasal una vez al día.
Uso en niños de edad comprendida entre 3 y 11 años
La dosis recomendada es de una pulverización en cada orificio nasal una vez al día.
En algunos pacientes, Mometasona furoato Teva comienza a aliviar los síntomas en las 12 horas siguientes a la primera dosis; sin embargo, no es probable que el efecto óptimo del tratamiento se observe antes de los primeros dos días. Por lo tanto, usted debe mantener un uso regular para alcanzar el efecto óptimo del tratamiento.
Si usted o su hijo presentan fiebre del heno muy intensa, su médico puede aconsejarle que comience a utilizar Mometasona furoato Teva unos días antes de empezar la estación polínica, ya que esto le ayudará a prevenir los síntomas de la fiebre del heno. Al final de la estación polínica sus síntomas de la fiebre del heno deben mejorar y el tratamiento puede entonces no ser necesario.
Pólipos nasales
Uso en adultos de más de 18 años de edad
La dosis inicial recomendada es de dos pulverizaciones en cada orificio nasal una vez al día.
- Si después de 5 a 6 semanas no se controlan los síntomas, la dosis puede incrementarse a dos pulverizaciones en cada orificio nasal dos veces al día. Una vez que se ha alcanzado el control de los síntomas, es posible que su médico le reduzca la dosis.
- Si no se observa mejoría de los síntomas después de 5-6 semanas administrándose dos veces al día, debe consultar con su médico para considerar tratamientos alternativos a Mometasona furoato Teva.
Preparación del pulverizador nasal para su uso
Mometasona furoato Teva pulverizador nasal tiene un tapón que protege la boquilla y la mantiene limpia. Recuerde quitarlo antes de utilizar el pulverizador y volverlo a poner después de usarlo.
Si va a utilizar el pulverizador por primera vez necesita “cebar” el frasco pulsando el pulverizador 10 veces hasta que se produzca una fina pulverización:
- Primero agite con cuidado el frasco.
- Ponga los dedos índice y corazón a ambos lados de la boquilla y el pulgar debajo del frasco. Noperfore el aplicador nasal.
- No apunte con la boquilla hacia usted y después presione con sus dedos para pulverizar 10 veces hasta que se produzca una pulverización fina (Figura 1) .
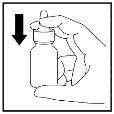
Figura 1
Si no ha usado su pulverizador nasal durante 14 días o más, necesita “cebar” de nuevo el frasco pulsando el pulverizador 2 veces hasta que se produzca una fina pulverización.
Cuánto tiempo dura su pulverizador nasal
A la dosis normal de dos pulverizaciones en cada orificio nasal una vez al día durante el tratamiento de fiebre del heno y rinitis pernne y pólipos nasales, este medicamento proporciona dosis suficientes para 15 días (si el frasco contiene 60 pulverizaciones), 30 días (si el frasco contiene 120 pulverizaciones) y 35 días (si el frasco contiene 140 pulverizaciones).
Cómo utilizar su pulverizador nasal
- Agite con cuidado el frasco y quite el tapón (Figura 2)
Tapón Boquilla Frasco |
Figura 2 |
- Suénese suavemente la nariz
- Tape un orificio nasal y ponga la boquilla en el otro orificio según se indica (Figura 3).
Incline ligeramente su cabeza hacia abajo, manteniendo el frasco en vertical.
Dirija la boquilla hacia el lateral de la fosa nasal, no hacia el centro (tabique nasal).
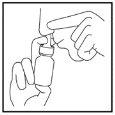
Figura 3
- Empiece a inspirar suave o lentamente por la nariz y mientras está inspirando pulverice el fino pulverizado en la nariz presionando UNA VEZ con los dedos (Figura 4).
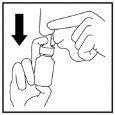
Figura 4
- Espire a través de la boca. Repita el paso 4 para inhalar un segundo pulverizado en el mismo orificio nasal, si procede.
- Quite la boquilla de este orificio nasal y espire por la boca.
- Repita los pasos 3 a 6 en el otro orificio nasal (Figura 5).
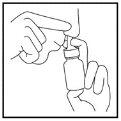
Figura 5
Después de utilizar el pulverizador, limpie la boquilla cuidadosamente con un pañuelo limpio o pañuelo de papel y ponga el tapón.
Limpieza de su pulverizador nasal
- Es importante que limpie regularmente su pulverizador nasal, de lo contrario puede que no funcione adecuadamente.
- Quite el tapón (Figura 6) y tire con cuidado de la boquilla (Figura 7).
Tapón |
Figura 6 | Boquilla |
|
- Lave el tapón (Figura 8) y la boquilla (Figura 9) con agua templada y luego aclare con agua corriente.
Tapón |
Figura 8 | Boquilla |
|
- No trate de desatascar el aplicador nasal mediante la inserción de una aguja u otro objeto puntiagudo ya que podría dañar el aplicador de forma que no se reciba la dosis correcta de medicamento.
- Ponga el tapón y la boquilla a secar en un lugar cálido.
Presione la boquilla sobre el frasco (Figura 10) y coloque el tapón (Figura 11).
Orificio central Boquilla Sistema de bombeo |
Figura 10 |
Figura 11 |
- El pulverizador necesitará ser cebado de nuevo con 2 pulverizaciones cuando se use por primera vez después de la limpieza (Figura 12).
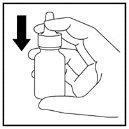
Figura 12
Siusa más MometasonafuroatoTeva del que debe
Si accidentalmente usa más del que debiera coménteselo a su médico.
Si utiliza esteroides durante un periodo de tiempo largo o en grandes cantidades puede, raramente, afectar a alguna de sus hormonas. En niños esto puede afectar al crecimiento y desarrollo.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico, o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida. Se recomienda llevar el envase y el prospecto del medicamento al profesional sanitario.
Si olvidó usar MometasonafuroatoTeva
Si olvidara usar su pulverizador nasal en el momento adecuado, utilícelo tan pronto como lo recuerde, siguiendo posteriormente con el ritmo normal de administración. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con MometasonafuroatoTeva
En algunos pacientes Mometasona furoato Teva debe empezar a aliviar los síntomas 12 horas después de la primera dosis; sin embargo, puede que el beneficio completo del tratamiento no se muestre hasta dos días después. Es muy importante que use su pulverizador nasal regularmente. No interrumpa su tratamiento incluso si se siente mejor a menos que su médico se lo indique.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Pueden ocurrir reacciones inmediatas de hipersensibilidad (alérgicas) después del uso de este medicamento. Estas reacciones pueden ser graves. Debe interrumpir el tratamiento con Mometasona furoato Teva y buscar ayuda médica inmediatamente si experimenta síntomas como:
- cara hinchada, lengua o faringe
- dificultad para tragar
- urticaria
- fatiga o dificultad para respirar
Si los pulverizadores nasales de corticosteroides se utilizan a dosis altas durante largos periodos de tiempo, se pueden producir efectos adversos, debido a que el medicamento se absorbe en el organismo.
Otros efectos adversos
La mayoría de las personas no tienen ningún problema después de usar el pulverizador nasal. Sin embargo, algunas personas después de utilizar Mometasona furoato Teva u otros pulverizadores nasales de corticosteroides pueden presentar:
Efectos adversos frecuentes (puede afectar hasta 1 de cada 10 personas)
- dolor de cabeza
- estornudos e irritación nasal/sensación de quemazón en la nariz
- hemorragia nasal[muy frecuente (puede afectar a más de 1 de cada 10 personas) en personas con pólipos nasales que estén administrándose dos pulverizaciones de Mometasona furoato Teva en cada orificio nasal dos veces al día.]
- dolor en la nariz o en la garganta
- úlceras en la nariz
- infección respiratoria
Frecuencia no conocida
- aumento de la presión ocular (glaucoma) y/o cataratas que pueden dar lugar a alteraciones visuales
- daño en el tabique de la nariz que separa los orificios nasales
- alteraciones del gusto y del olfato
- dificultad para respirar y/o fatiga
- Visión borrosa
Otrosefectos adversos en niños y adolescentes
Se espera que la frecuencia y el tipo de reacciones adversas en niños sean las mismas que en adultos. El uso prolongado de corticosteroides por vía nasal puede causar ciertos efectos adversos, como un enlentecimiento del crecimiento en niños. En tratamientos prolongados con corticosteroides por vía nasal se recomienda controlar la altura del niño cada cierto tiempo y si se observan cambios, informar a su médico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Mometasona furoato Teva
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el frasco y en en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25ºC. No congelar.
El pulverizador debe utilizarse dentro de las 8 semanas desde su primera administración.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de MometasonafuroatoTeva 50 microgramos suspensión para pulverización nasal
- El principio activo es furoato de mometasona. Cada pulverización (0,1 ml) contiene 50 microgramos de furoato de mometasona (como monohidrato).
- Los demás componentes son celulosa microcristalina y carmelosa sódica, glicerol, citrato de sodio, ácido cítrico monohidrato, polisorbato 80, cloruro de benzalconio (ver sección 2) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Mometasona furoato Teva es una suspensión para pulverización nasal.
Cada frasco contiene 60, 120 ó 140 pulverizaciones.
Los frascos que contienen 60 o 120 pulverizaciones se presentan en envases con un único frasco.
Los frascos que contienen 140 pulverizaciones se presentan en envases con 1, 2 ó 3 frascos.
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Teva Pharma, S.L.U.
C/Anabel Segura, 11 Edificio Albatros B, 1ª Planta
28108 Alcobendas, Madrid (España)
Responsable de la fabricación
Teva Czech Industries s.r.o
Ostravská 305/29,
Komárov 747 70 Opava
República Checa
o
Merckle GmbH
Ludwig-Merckle-Str. 3
89143 Blaubeuren, Germany
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria Mometason Ratiopharm 50 mikrogramm/Spruhstoß Nasenspray, Suspension
Alemania Mometasonfuroat AbZ 50 Mikrogramm/Sprühstoß Nasenspray, Suspension
Bélgica Mometasone Teva 50 microgram per verstuiving, neusspray, suspensie
Dinamarca Mometasonfuroat Teva
España Mometasona furoato Teva 50 microgramos suspensión para pulverización nasalFinlandia Momesonex 50 mikrog/annos nenäsumute, suspensie
Francia MOMETASONE TEVA 50 microgrammes/dose, suspension pour pulvérisation nasale.
Hungría Nasotasone 50 mcg szuszpenziós adagolt orrspray
Italia Mometasone TEVA
Lituania Mometasone Teva 50 mikrogramu/dozeje nosies purškalas (suspensija)
Países Bajos Mometasonfuroaat Teva 50 microgram/verstuiving, neusspray, suspensie
Polonia Pronasal
Portugal Mometasona Teva, 50 μg/dose, Suspensão para pulverização nasal
Suecia Mometasone Teva 50 mikrogram/dos nässpray, suspension
Fecha de la última revisión de este prospecto: Febrero 2024
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
Puede acceder a información detallada y actualizada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el cartonaje. También puede acceder a esta información en la siguiente dirección de internet: https://cima.aemps.es/cima/dochtml/p/77974/P_77974.html
- País de registro
- Precio medio en farmacia8.99 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MOMETASONA FUROATO TEVA 50 MICROGRAMOS SUSPENSION PARA PULVERIZACION NASALForma farmacéutica: PRODUCTO USO NASAL, 50 microgramos/pulverizaciónPrincipio activo: mometasoneFabricante: Laboratorios Cinfa S.A.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 50 microgramosPrincipio activo: mometasoneFabricante: Aristo Pharma GmbhRequiere recetaForma farmacéutica: PRODUCTO USO NASAL, 50 microgramos/pulsacionPrincipio activo: mometasoneFabricante: Laboratorios Alter S.A.Requiere receta
Médicos online para MOMETASONA FUROATO TEVA 50 MICROGRAMOS SUSPENSION PARA PULVERIZACION NASAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MOMETASONA FUROATO TEVA 50 MICROGRAMOS SUSPENSION PARA PULVERIZACION NASAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






 Figura 7
Figura 7
 Figura 9
Figura 9








