
MIRTAZAPINA AUROVITAS SPAIN 45 MG COMPRIMIDOS BUCODISPERSABLES EFG

Cómo usar MIRTAZAPINA AUROVITAS SPAIN 45 MG COMPRIMIDOS BUCODISPERSABLES EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Mirtazapina Aurovitas Spain 45 mg comprimidos bucodispersables EFG
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Mirtazapina Aurovitas Spain y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Mirtazapina Aurovitas Spain
- Cómo tomar Mirtazapina Aurovitas Spain
- Posibles efectos adversos
- Conservación de Mirtazapina Aurovitas Spain
- Contenido del envase e información adicional
1. Qué es Mirtazapina Aurovitas Spain y para qué se utiliza
Mirtazapina Aurovitas Spain pertenece al grupo de medicamentos llamados antidepresivos.
Mirtazapina se utiliza para tratar la depresión en adultos.
Se requieren de 1 a 2 semanas antes de que mirtazapina empiece a hacer efecto. Después de 2 a 4 semanas usted puede empezar a encontrarse mejor. Debe consultar a su médico si empeora o si no mejora después de 2 a 4 semanas. Para más información ver la sección 3 “Cuándo puede esperar encontrarse mejor”.
2. Qué necesita saber antes de empezar a tomar Mirtazapina Aurovitas Spain
No tome o consulte a su médico antes de empezar a tomarMirtazapinaAurovitas Spain
- si es alérgico al principio activo o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). En ese caso, consulte a su médico lo antes posible antes de tomar mirtazapina.
- si está tomando o ha tomado recientemente (en las dos últimas semanas) medicamentos llamados inhibidores de la monoaminooxidasa (IMAOs).
- si ha sufrido alguna vez una erupción cutánea intensa o descamación de la piel, ampollas o llagas en la boca después de tomar mirtazapina u otros medicamentos.
Tenga especial precaución con Mirtazapina Aurovitas Spain
Se han notificado con el uso de mirtazapina reacciones cutáneas graves, como síndrome de Stevens-Johnson (SSJ), necrólisis epidérmica tóxica (NET) y reacciones medicamentosa con eosinofilia y síntomas sistémicos (DRESS). Interrumpa su uso y busque atención médica inmediatamente si nota alguno de los síntomas descritos en la sección 4 en relación con estas reacciones cutáneas graves.
Si ha sufrido alguna vez reacciones cutáneas graves, no debe reiniciarse el tratamiento con mirtazapina.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Mirtazapina Aurovitas Spain.
Si usted está tomando medicamentos que contienen buprenorfina. El uso de estos medicamentos junto con mirtazapina puede provocar síndrome serotoninérgico, una enfermedad potencialmente mortal (ver Otros medicamentos y Mirtazapina Aurovitas Spain).
Niños y adolescentes
Mirtazapina no debe utilizarse normalmente en el tratamiento de niños y adolescentes menores de 18 años porque no se ha demostrado su eficacia. A la vez, debe saber que en pacientes menores de 18 años existe un mayor riesgo de efectos adversos como intentos de suicidio, ideación suicida y hostilidad (predominantemente agresividad, comportamiento de confrontación e irritación) cuando toman esta clase de medicamentos. Pese a ello, su médico puede prescribir mirtazapina a pacientes menores de 18 años cuando decida qué es lo más conveniente para el paciente. Si su médico ha prescrito mirtazapina a un paciente menor de 18 años y desea discutir esta decisión, por favor, vuelva a su médico. Debe informar a su médico si aparecen o empeoran alguno de los síntomas que se detallan anteriormente en pacientes menores de 18 años que están tomando mirtazapina. Además, todavía no se conocen los efectos a largo plazo en lo que a seguridad se refiere y relacionados con el crecimiento, la madurez y el desarrollo cognitivo y la conducta de mirtazapina en este grupo de edad. También se ha observado con mayor frecuencia un considerable aumento de peso en este grupo de edad cuando son tratados con mirtazapina, en comparación con los adultos.
Ideas de suicidio y empeoramiento de la depresión
Si se encuentra deprimido puede que a veces tenga ideas de hacerse daño a sí mismo o de suicidarse. Esto podría empeorar cuando empieza a tomar los antidepresivos por primera vez, ya que estos medicamentos tardan en hacer efecto, normalmente dos semanas o a veces más.
Podría ser más propenso a pensar de esta manera:
- Si previamente ha tenido pensamientos suicidas o de hacerse daño a sí mismo.
- Si es un adulto joven. La información de los ensayos clínicos ha mostrado un riesgo aumentado de comportamiento suicida en adultos menores de 25 años con trastornos psiquiátricos y que estaban en tratamiento con antidepresivos.
→ Si tiene pensamiento de hacerse daño a sí mismo o suicidarse en algún momento, consulte a su médico o vaya a un hospital inmediatamente.
Puede ser útil decirle a un familiar o amigo cercanoque se encuentra deprimido, y pedirle que se lea este prospecto. Puede pedirle que le diga si cree que su depresión está empeorando, o si está preocupado por cambios en su comportamiento.
Asimismo, tenga especial cuidado con mirtazapina:
- si tiene o ha tenido alguna vez uno de los siguientes trastornos:
→ Informe a su médico sobre estas situaciones antes de tomar mirtazapina, si no lo ha hecho ya.
- convulsiones(epilepsia). Si aparecen convulsiones o sus convulsiones son más frecuentes, deje de tomar mirtazapina y contacte con su médico inmediatamente;
- enfermedades del hígado, incluyendo ictericia. Si aparece ictericia, deje de tomar mirtazapina y contacte con su médico inmediatamente;
- enfermedades de los riñones;
- enfermedades del corazón, o presión arterial baja;
- esquizofrenia. Si los síntomas psicóticos, como pensamientos paranoicos, se vuelven más frecuentes o se agravan, contacte inmediatamente con su médico;
- depresión maniaca(se alternan períodos de animación/hiperactividad y períodos de depresión). Si comienza a sentirse animado o sobrexcitado, deje de tomar mirtazapina y contacte con su médico inmediatamente;
- diabetes(podría necesitar ajustar su dosis de insulina u otros medicamentos antidiabéticos);
- enfermedades de los ojos, como aumento de la presión en el ojo (glaucoma);
- dificultadpara orinar, que podría deberse a un aumento del tamaño de la próstata;
- ciertos tipos de enfermedades del corazónque pueden cambiar el ritmo de su corazón, un ataque reciente al corazón, un fallo del corazón, o toma de ciertos medicamentos que pueden afectar el ritmo del corazón.
- si aparecen signos de infección, tales como fiebre alta, dolor de garganta y llagas en la boca.
→ Deje de tomar mirtazapinay contacte con su médico inmediatamente para realizar un análisis de sangre. En raras ocasiones estos síntomas pueden ser signos de alteraciones en la producción de células sanguíneas en la médula ósea. Aunque raros, estos síntomas aparecen en su mayor parte a las 4-6 semanas de tratamiento.
- si es una persona de edad avanzada podría ser más sensible a los efectos adversos de los medicamentos antidepresivos.
Otros medicamentos y Mirtazapina Aurovitas Spain
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
No tome Mirtazapina Aurovitas Spainjunto con:
- inhibidores de la monoaminooxidasa(inhibidores de la MAO). Asimismo, no tome mirtazapina durante las dos semanas después de haber dejado de tomar inhibidores de la MAO. Si deja de tomar mirtazapina, tampoco tome inhibidores de la MAO durante las siguientes dos semanas.
Ejemplos de inhibidores de la MAO son moclobemida, tranilcipromina (ambos son antidepresivos) y selegilina (para la enfermedad de Parkinson).
Tenga cuidado sitoma Mirtazapina Aurovitas Spain junto con:
- antidepresivos tales comoinhibidores selectivos de la recaptación de serotonina (ISRSs), venlafaxina yL-triptófano o triptanos(utilizados para la migraña), tramadol(para el dolor), linezolid(un antibiótico), litio(utilizado para tratar algunos trastornos psiquiátricos), azul de metileno (utilizado para tratar niveles altos de metahemoglobina en la sangre) y preparados a base de Hierba de San Juan – Hypericum perforatum(planta medicinal para la depresión). En casos muy raros, mirtazapina solo o junto con estos medicamentos, puede dar lugar al llamado síndrome serotoninérgico. Algunos de los síntomas de este síndrome son: fiebre inexplicable, sudoración, palpitaciones, diarrea, contracciones musculares (incontrolables), escalofríos, reflejos exagerados, agitación, cambios de humor y pérdida de consciencia. Si presenta una combinación de estos síntomas, consulte a su médico inmediatamente.
- el antidepresivo nefazodona. Puede aumentar la cantidad de mirtazapina en sangre. Informe a su médico si está tomando este medicamento. Podría ser necesario disminuir la dosis de mirtazapina o aumentarla nuevamente al dejar de tomar nefazodona.
- medicamentos para la ansiedad o el insomniocomo las benzodiazepinas;
- medicamentos para la esquizofreniacomo la olanzapina;
- medicamentos para las alergiascomo la cetirizina;
- medicamentos para el dolor intensocomo la morfina.
En combinación con estos medicamentos, mirtazapina puede aumentar la somnolencia causada por estos medicamentos.
- medicamentos para infecciones; medicamentos para infecciones bacterianas (como la eritromicina), medicamentos para infecciones por hongos (como el ketoconazol), los medicamentos para el VIH/SIDA (inhibidores de la proteasa del VIH) y medicamentos para la úlcera de estómago(como la cimetidina).
Si se toman junto con mirtazapina, estos medicamentos pueden aumentar la cantidad de mirtazapina en sangre. Informe a su médico si está tomando estos medicamentos. Podría ser necesario disminuir la dosis de mirtazapina, o aumentarla nuevamente al dejar de tomar estos medicamentos.
- medicamentos para la epilepsiacomo la carbamazepina y la fenitoína;
- medicamentos para la tuberculosiscomo la rifampicina.
Si se toman junto con mirtazapina, estos medicamentos pueden reducir la cantidad de mirtazapina en sangre. Informe a su médico si está tomando estos medicamentos. Podría ser necesario aumentar la dosis de mirtazapina, o disminuirla nuevamente al dejar de tomar estos medicamentos.
- medicamentos para prevenir la coagulación de la sangrecomo la warfarina.
Mirtazapina puede aumentar los efectos de la warfarina en la sangre. Informe a su médico si está tomando este medicamento. En caso de tomarlos a la vez, se recomienda que el médico le haga controles en sangre.
- medicamentos que pueden afectar el ritmo del corazón, como ciertos antibióticos y algunos antipsicóticos.
Algunos medicamentos pueden aumentar los efectos secundarios de mirtazapina y a veces pueden causar reacciones muy graves. No tome ningún otro medicamento mientras esté tomando mirtazapina sin consultar primero con su médico, especialmente:
- medicamentos que contienen buprenorfina. Estos medicamentos pueden interactuar con mirtazapina y puede experimentar síntomas como contracciones involuntarias y rítmicas de los músculos, incluyendo los músculos que controlan el movimiento del ojo, agitación, alucinaciones, coma, sudoración excesiva, temblor, exageración de los reflejos, aumento de la tensión muscular, temperatura corporal por encima de 38oC. Comuníquese con su médico cuando experimente estos síntomas.
Toma deMirtazapinaAurovitas Spaincon alimentos, bebidas y alcohol
Puede sentirse somnoliento si bebe alcohol mientras esté en tratamiento con mirtazapina.
Se recomienda no beber nada de alcohol.
Pueden tomar mirtazapina con o sin alimentos.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
La experiencia limitada de la administración de mirtazapina a mujeres embarazadas no indica un aumento de riesgo. Sin embargo, debe tenerse cuidado si se usa durante el embarazo.
Si usa mirtazapina hasta el parto, o poco antes, su hijo será examinado para detectar posibles efectos adversos.
Cuando se toma durante el embarazo, medicamentos similares (ISRS) pueden aumentar el riesgo de una afección grave en el bebé, denominada hipertensión pulmonar persistente del recién nacido (HPPRN), lo que hace que el bebé respire más rápido y parezca azulado. Estos síntomas comienzan normalmente durante las primeras 24 horas después de que nazca el bebé. Si esto le sucede al bebé, debe consultar a su matrona o médico de inmediato.
Conducción y uso de máquinas
Mirtazapina Aurovitas Spain puede afectar a su capacidad de concentración o estado de alerta. Asegúrese de que sus facultades no están afectadas antes de conducir o utilizar maquinaria. Si su médico ha recetado mirtazapina a un paciente menor de 18 años asegúrese de que la concentración y la alerta no se ven afectadas antes de circular (por ejemplo, en bicicleta).
MirtazapinaAurovitas Spain contiene aspartamo
Este medicamento contiene 9 mg de aspartamo en cada comprimido bucodispersable.
El aspartamo contiene una fuente de fenilalanina que puede ser perjudicial en caso de padecer fenilcetonuria (FCN), una enfermedad genética rara en la que la fenilalanina se acumula debido a que el organismo no es capaz de eliminarla correctamente.
3. Cómo tomar Mirtazapina Aurovitas Spain
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cuánto tomar
La dosis inicial recomendada es de 15 mg o 30 mg al día.Su médico puede recomendarle aumentar la dosis tras unos días hasta la cantidad que sea mejor para usted (entre 15 y 45 mg al día). Normalmente la dosis es la misma para todas las edades. Sin embargo, si es una persona de edad avanzada o si padece una enfermedad del riñón o del hígado, su médico podría cambiar la dosis.
Cuándo tomar Mirtazapina Aurovitas Spain
→ Tome Mirtazapina Aurovitas Spain a la misma hora cada día.
Es mejor tomar la dosis de mirtazapina de una sola vez antes de acostarse. Sin embargo, su médico puede recomendarle que divida su dosis de mirtazapina por la mañana y por la noche antes de acostarse. La dosis más alta debe tomarse antes de acostarse.
Tome el comprimido bucodispersable de la siguiente manera:
Los comprimidos se toman por vía oral.
- No aplaste el comprimido bucodispersable
Para evitar que el comprimido bucodispersable se aplaste, no presionar el alveolo (Figura A).
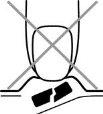
Fig. A
- Separe un alveolo
Cada blíster contiene seis alveolos, que están separados por perforaciones. Separe un alveolo siguiendo las líneas perforadas (Figura 1).
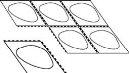
Fig. 1
- Retire la lámina
Retire cuidadosamente la lámina, comenzando por la esquina marcada con una flecha (Figuras 2 y 3).
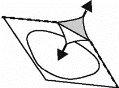
Fig. 2
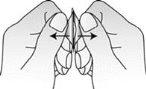
Fig. 3
- Saque el comprimido bucodispersable
Saque el comprimido bucodispersable con las manos secas y póngalo en la lengua (Figura 4).
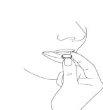
Fig. 4
Se deshará rápidamente y puede tragarse sin agua.
Cuándo puede esperar encontrarse mejor
Normalmente mirtazapina empezará a hacer efecto después de 1 o 2 semanas y después de 2 a 4 semanas podría empezar a encontrarse mejor. Es importante que durante las primeras semanas de tratamiento hable con su médico sobre los efectos de mirtazapina:
Entre 2 y 4 semanas después de haber empezado a tomar mirtazapina, hable con su médico sobre cómo le ha afectado este medicamento.
Si todavía no se encuentra mejor, su médico podría recetarle una dosis mayor. En ese caso, hable nuevamente con su médico después de otras 2-4 semanas. Normalmente necesitará tomar mirtazapina hasta que los síntomas de depresión hayan desaparecido durante 4-6 meses.
Si toma másMirtazapinaAurovitas Spainde la que debe
Si usted o alguien toma demasiada mirtazapina, consulte a un médico inmediatamente.
Los síntomas más probables de una sobredosis de mirtazapina (sin otros medicamentos o alcohol) son somnolencia, desorientación y palpitaciones. Los síntomas de una posible sobredosis pueden incluir cambios en el ritmo de su corazón (latido rápido, irregular) y/o desfallecimiento, que podrían ser síntomas de una enfermedad potencialmente mortal conocida como Torsades de Pointes.
En caso de sobredosis o ingestión accidental, consulte inmediatamente con su médico o farmacéutico o acuda al hospital más cercano. También puede llamar al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomarMirtazapinaAurovitas Spain
Si tiene que tomar su dosis una vez al día
- No tome una dosis doble para compensar las dosis olvidadas. Tome la siguiente dosis a la hora habitual.
Si tiene que tomar su dosis dos veces al día
- Si ha olvidado la dosis de la mañana, simplemente tómesela junto con la dosis de la noche.
- Si ha olvidado la dosis de la noche, no la tome a la mañana siguiente; sáltesela y continúe con sus dosis normales por la mañana y por la noche.
- Si ha olvidado ambas dosis, no intente recuperarlas. Sáltese ambas dosis y al día siguiente continúe con la dosis normal por la mañana y por la noche.
Si interrumpe el tratamiento conMirtazapinaAurovitas Spain
→ Deje de tomar mirtazapina sólo si lo consulta con su médico.
Si lo deja demasiado pronto, la depresión podría reaparecer. Cuando se encuentre mejor, hable con su médico. Su médico decidirá cuándo puede dejar el tratamiento.
No deje de tomar mirtazapina bruscamente, aun cuando la depresión haya desaparecido. Si deja de tomar mirtazapina de forma brusca, puede sentirse enfermo, mareado, agitado o ansioso y tener dolores de cabeza.
Estos síntomas pueden evitarse dejando el tratamiento gradualmente. Su médico le indicará cómo disminuir la dosis gradualmente.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si experimenta alguno de los siguientes efectos adversos, deje de tomarMirtazapina Aurovitas Spaine informe inmediatamente a su médico.
Poco frecuentes(pueden afectar hasta a 1 de cada 100 personas):
- sentimiento de euforia exagerada (manía).
Raros(pueden afectar hasta a 1 de cada 1.000 personas):
- coloración amarilla de los ojos o la piel; puede sugerir alteraciones en el funcionamiento del hígado (ictericia).
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- signos de infección tales como fiebre alta inexplicable y repentina, dolor de garganta y llagas en la boca (agranulocitosis). En casos raros, mirtazapina puede provocar alteraciones en la producción de células sanguíneas (depresión de la médula ósea). Algunas personas se vuelven menos resistentes a las infecciones porque mirtazapina puede provocar una disminución temporal de los glóbulos blancos de la sangre (granulocitopenia). En casos raros, Mirtazapina Aurovitas Spain también puede provocar una disminución de los glóbulos rojos y blancos y de las plaquetas (anemia aplásica), una disminución de las plaquetas (trombocitopenia) o un aumento en el número de glóbulos blancos en sangre (eosinofilia).
- ataque epiléptico (convulsiones).
- combinación de síntomas como fiebre inexplicable, sudoración, palpitaciones, diarrea, contracciones musculares (incontrolables), escalofríos, reflejos exagerados, agitación, cambios de humor y pérdida de consciencia y aumento de la pruducción de saliva. En casos muy raros, estos síntomas pueden ser señales de un trastorno llamado síndrome serotoninérgico.
- pensamientos de hacerse daño a uno mismo o de suicidio.
- reacciones graves en la piel (síndrome de Stevens-Johnson o necrólisis epidérmica tóxica).
- parches rojos en el tronco, como máculas circunscritas o circulares, a menudo con ampollas en el centro, desprendimiento de la piel, úlceras en la boca, la garganta, la nariz, los genitales y los ojos. Estos eritemas cutáneos graves pueden ir precedidos de fiebre y síntomas gripales (síndrome de Stevens-Johnson, necrólisis epidérmica tóxica).
- eritema generalizado, temperatura corporal elevada y aumento de tamaño de los ganglios linfáticos (síndrome DRESS o síndrome de hipersensibilidad medicamentosa).
Otros posibles efectos adversos con mirtazapina son:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- aumento del apetito y aumento de peso
- somnolencia
- dolor de cabeza
- boca seca
Frecuentes(pueden afectar hasta a 1 de cada 10 personas):
- letargia
- mareo
- temblores o temblor
- náuseas
- diarrea
- vómitos
- estreñimiento
- urticaria o erupciones en la piel (exantema)
- dolores en las articulaciones (artralgia) o músculos (mialgia)
- dolor de espalda
- mareo o desmayo al levantarse rápidamente (hipotensión ortostática)
- hinchazón (normalmente en tobillos o pies) debido a retención de líquidos (edema)
- cansancio
- sueños vívidos
- confusión
- ansiedad
- dificultades para dormir
- problemas de memoria, que en la mayoría de los casos se resolvieron cuando se suspendió el tratamiento.
Poco frecuentes(pueden afectar hasta a 1 de cada 100 personas):
- sensación extraña en la piel por ejemplo quemazón, pinchazos, cosquilleo u hormigueo (parestesia)
- piernas inquietas
- desmayos (síncope)
- sensación de adormecimiento de la boca (hipoestesia oral)
- tensión baja
- pesadillas
- agitación
- alucinaciones
- incapacidad para mantenerse quieto
Raros(pueden afectar hasta a 1 de cada 1.000 personas):
- tics o contracciones musculares (mioclono)
- agresión
- dolor abdominal y náuseas; esto puede indicar inflamación del páncreas (pancreatitis)
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- sensaciones anormales en la boca (parestesia oral)
- hinchazón en la boca (edema bucal)
- hinchazón por todo el cuerpo (edema generalizado)
- hinchazón localizada
- hiponatremia
- secreción inadecuada de hormona antidiurética
- reacciones graves en la piel (dermatitis bullosa, eritema multiforme)
- andar dormido (sonambulismo)
- problema del habla
- aumento de los niveles de creatina cinasa en la sangre
- dificultad para orinar (retención urinaria)
- dolor muscular, rigidez y/o debilidad, oscurecimiento o decoloración de la orina (rabdomiólisis)
- aumento de los niveles de la hormona prolactina en la sangre (hiperprolactinemia, incluyendo síntomas como agrandamiento de los senos y/o secreción lechosa por el pezón)
- erección dolorosa y prolongada del pene.
Otros efectos adversos en niños y adolescentes
En niños menores de 18 años se han observado frecuentemente los siguientes efectos adversos en ensayos clínicos: aumento de peso considerable, urticaria y aumento de los triglicéridos en la sangre.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Mirtazapina Aurovitas Spain
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en el blíster después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación .
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de MirtazapinaAurovitas Spain
- El principio activo es mirtazapina.
Mirtazapina Aurovitas Spain 45 mg comprimidos bucodispersables contiene 45 mg de mirtazapina por comprimido.
- Los demás componentes son: crospovidona (tipo B), manitol, celulosa microcristalina, aspartamo, sílice coloidal anhidra, estearato de magnesio, aroma a guaraná de fresa [maltodextrina, propilenglicol, aromas artificiales, ácido acético] y aroma a menta [sabores artificiales, almidón de maíz].
Aspecto del producto y contenido del envase
Comprimido bucodispersable.
Mirtazapina Aurovitas Spain 45 mg comprimidos bucodispersables:
Comprimidos bucodispersables blancos, redondos, con un borde circular en relieve, marcados con "38" en un lado y "A" en el otro lado.
Blíster perforado divisible en dosis individuales: poliamida/Aluminio/PVC/Papel/Poliéster.
Tamaños de envase:
45 mg: 30 comprimidos bucodispersables
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Aurovitas Spain, S.A.U.
Avda. de Burgos, 16-D
28036 Madrid
España
Telf.: 91 630 86 45
Fax: 91 630 26 64
Responsable de la fabricación
APL Swift Services (Malta) Limited
HF26, Half Far Industrial Estate, Hal Far,
Birzebbuggia, BBG 3000
Malta
O
Generis Farmacêutica, S.A.
Rua João de Deus, 19
2700-487 Amadora
Portugal
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
República Checa Mirtazapine Aurovitas
Malta Mirtazapine Aurobindo 45 mg orodispersible tablets
Polonia Mirtazapine Aurovitas
España Mirtazapina Aurovitas Spain 45 mg comprimidos bucodispersables EFG
Portugal Mirtazapina Aurovitas
Fecha de la última revisión de este prospecto:Septiembre 2021
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es)
- País de registro
- Precio medio en farmacia25.57 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MIRTAZAPINA AUROVITAS SPAIN 45 MG COMPRIMIDOS BUCODISPERSABLES EFGForma farmacéutica: COMPRIMIDO, 15 mgPrincipio activo: mirtazapinaFabricante: Laboratorios Alter S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 30 mgPrincipio activo: mirtazapinaFabricante: Laboratorios Alter S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 15 mgPrincipio activo: mirtazapinaFabricante: Almus Farmaceutica S.A.U.Requiere receta
Médicos online para MIRTAZAPINA AUROVITAS SPAIN 45 MG COMPRIMIDOS BUCODISPERSABLES EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MIRTAZAPINA AUROVITAS SPAIN 45 MG COMPRIMIDOS BUCODISPERSABLES EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











