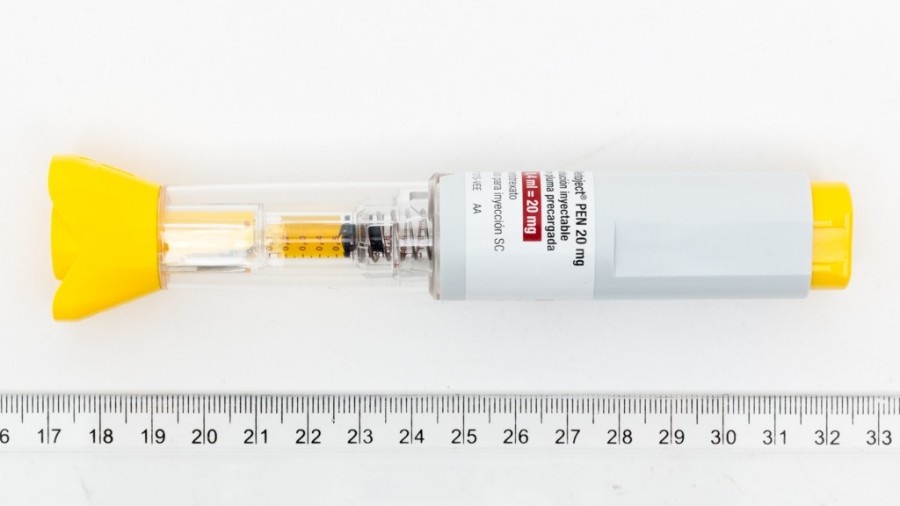
METHOFILL PEN 20 MG/0,40 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA EFG

Cómo usar METHOFILL PEN 20 MG/0,40 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Methofill Pen 20 mg/0,40 ml solución inyectable en pluma precargada EFG
metotrexato
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Methofill Pen y para qué se utiliza
- Qué necesita saber antes de empezar a usar Methofill Pen
- Cómo usar Methofill Pen
- Posibles efectos adversos
- Conservación de Methofill Pen
- Contenido del envase e información adicional
1. Qué es Methofill Pen y para qué se utiliza
Este medicamento está indicado para el tratamiento de:
- la artritis reumatoide activa en pacientes adultos,
- formas poliartríticas de artritis idiopática juvenil activa severa, cuando la respuesta a los antiinflamatorios no esteroideos (AINE) no ha sido adecuada,
- la psoriasis moderada a grave en pacientes adultos, y la artritis psoriásica grave en adultos,
- la enfermedad de Crohn leve a moderada en pacientes adultos cuando no es posible un tratamiento adecuado con otros medicamentos.
La artritis reumatoide (AR) es una enfermedad crónica del colágeno, que se caracteriza por la inflamación de las membranas sinoviales (membranas de las articulaciones). Estas membranas producen un líquido que actúa como lubricante en muchas articulaciones. La inflamación provoca el engrosamiento de la membrana y la hinchazón de la articulación.
La artritis juvenil afecta a niños y adolescentes menores de 16 años. Las formas poliartríticas están indicadas si hay afectación de 5 o más articulaciones en los primeros 6 meses de la enfermedad.
La psoriasis es una enfermedad crónica y frecuente de la piel, que se caracteriza por unas manchas rojas cubiertas por escamas gruesas, secas, plateadas y adherentes.
La artritis psoriásica es un tipo de artritis con lesiones psoriásicas en la piel y las uñas, especialmente en las articulaciones de los dedos de las manos y pies.
Este medicamento modifica y enlentece la evolución de la enfermedad.
La enfermedad de Crohn es un tipo de enfermedad inflamatoria del intestino que puede afectar a cualquier parte del tracto gastrointestinal causando síntomas tales como dolor abdominal, diarrea, vómitos o pérdida de peso.
2. Qué necesita saber antes de empezar a usar Methofill Pen
No use Methofill Pen
- si es alérgico al metotrexato o a alguno de los demás componentes de este medicamento (incluidos en la sección 6),
- si tiene enfermedad hepática o renal grave o enfermedades de la sangre,
- si toma habitualmente grandes cantidades de alcohol,
- si tiene una infección grave, como tuberculosis, VIH u otros síndromes de inmunodeficiencia,
- si tiene úlceras bucales, úlcera gástrica o úlcera intestinal,
- si está embarazada o en periodo de lactancia (ver sección «Embarazo, lactancia y fertilidad»),
- si recibe vacunas elaboradas con microorganismos atenuados al mismo tiempo.
Advertencias y precauciones
El metotrexato puede hacer que la piel sea más sensible a la luz solar. Evite el sol intenso y no utilice camas de bronceado ni lámparas ultravioletas sin consejo médico. Para proteger la piel del sol intenso, lleve ropa adecuada o utilice un protector solar con un factor de protección alto.
Consulte a su médico o farmacéutico antes de empezar a usar este medicamento si:
- tiene una edad avanzada o siente por lo general malestar y debilidad,
- tiene problemas con el funcionamiento del hígado,
- tiene problemas de deshidratación (pérdida de líquidos).
Se ha notificado con metotrexato hemorragia pulmonar aguda en pacientes con enfermedad reumatológica subyacente. Si observa sangre al escupir o toser, se debe poner en contacto de forma inmediata con su médico.
Medidas de precaución especiales para el tratamiento con Methofill Pen
El metotrexato afecta de manera temporal a la producción de espermatozoides y óvulos, lo que es reversible en la mayoría de los casos. El metotrexato puede causar abortos y defectos de nacimiento graves. Si usted es mujer, debe evitar tener un hijo si está recibiendo metotrexato y durante al menos 6 meses después de finalizar su tratamiento con metotrexato. Si usted es hombre, debe evitar tener un hijo si está recibiendo metotrexato y durante al menos 3 meses después de finalizar su tratamiento. Ver también sección «Embarazo, lactancia y fertilidad».
Pruebas de seguimiento y medidas de seguridad recomendadas:
Incluso cuando se administra este medicamento a dosis bajas, se pueden producir reacciones adversas graves. Para poder detectarlos a tiempo, es necesario que su médico le haga analíticas y chequeos.
Antes de iniciar el tratamiento con Methofill Pen:
Antes de iniciar el tratamiento, le harán análisis de sangre para comprobar que tenga suficientes células sanguíneas, y pruebas para comprobar el funcionamiento del hígado y averiguar si tiene hepatitis. Además se comprobará la concentración de la albúmina sérica (una proteína de la sangre), el estado de la hepatitis (infección del hígado) y el funcionamiento de los riñones. El médico también puede decidir realizar otras pruebas hepáticas, algunas de las cuales pueden ser imágenes de su hígado y otras pueden requerir la toma de una pequeña muestra de tejido del hígado para examinarlo más de cerca. Su médico también comprobará si tiene tuberculosis (una enfermedad infecciosa junto con pequeños nódulos en el tejido afectado) y le harán una radiografía de tórax o una prueba de función pulmonar.
Durante el tratamiento:
Su médico le realizarán los siguientes análisis:
- examen de la cavidad oral y la faringe para detectar cambios en la membrana mucosa, como inflamación o ulceraciónanálisis de sangre/
- recuento sanguíneo con número de células sanguíneas y medición de los niveles séricos de metotrexato
- análisis de sangre para controlar la función hepática
- pruebas de imagen para controlar el estado del hígado
- pequeña muestra de tejido tomada del hígado para examinarla más de cerca
- análisis de sangre para controlar la función renal
- revisión del aparato respiratorio y si fuera necesario la prueba de la función pulmonar
Es muy importante que se presente a estas pruebas programadas.
Si los resultados de alguna de estas pruebas son llamativos, su médico ajustará su tratamiento en consecuencia.
El metotrexato puede afectar al sistema inmunitario y a los resultados de la vacunación. También puede afectar al resultado de las pruebas inmunológicas. Puede reactivar las infecciones crónicas inactivas (como herpes zoster [“culebrillas”], tuberculosis, hepatitis B o C). Durante el tratamiento con este medicamento no debe recibir vacunas elaboradas con microorganismos atenuados.
Durante el tratamiento con metotrexato pueden reaparecer dermatitis inducidas por la radiación y quemaduras solares (reacciones de memoria). Las lesiones psoriásicas pueden intensificarse durante la radiación UV y la administración simultánea de metotrexato.
Puede producirse un aumento del tamaño de los nódulos linfáticos (linfoma) y, en dicho caso, el tratamiento debe suspenderse.
La diarrea puede ser un efecto adverso de este medicamento que requiere la suspensión del tratamiento. Si tiene diarrea, hable con su médico.
Se han notificado ciertos trastornos cerebrales (encefalopatía/leucoencefalopatía) en pacientes con cáncer tratados con metotrexato. No puede descartarse la aparición de estos efectos adversos cuando el metotrexato se usa para tratar otras enfermedades.
Si usted, su pareja o su cuidador notan la aparición o un empeoramiento de síntomas neurológicos, como debilidad muscular general, alteraciones de la visión, cambios en el pensamiento, la memoria y la orientación que generan confusión y cambios en la personalidad, contacte con su médico inmediatamente porque estos pueden ser síntomas de una infección cerebral grave muy rara denominada leucoencefalopatía multifocal progresiva (LMP).
Pacientes de edad avanzada
Los pacientes de edad avanzada en tratamiento con metotrexato deben ser vigilados estrechamente por un médico para poder detectar lo antes posible los posibles efectos secundarios.
El deterioro de la función hepática y renal relacionado con la edad, así como las bajas reservas corporales de ácido fólico en la tercera edad, requieren una dosis relativamente baja de metotrexato.
Uso de Methofill Pencon otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Tenga esto en cuenta también para medicamentos que pueda tomar en el futuro.
El efecto del tratamiento puede verse afectado si este medicamento se administra al mismo tiempo que ciertos medicamentos:
- Antibióticostales como: las tetraciclinas, el cloranfenicol, los antibióticos no absorbibles de amplio espectro, las penicilinas, los glucopéptidos, las sulfonamidas, la ciprofloxacina y la cefalotina (medicamentos para prevenir o combatir ciertas infecciones).
- Amoxicilina(las penicilinas pueden reducir la excreción de metotrexato causando un potencial aumento de los efectos secundarios).
- Antiinflamatorios no esteroideoso los salicilatos(medicamentos para el dolor o inflamación como el ácido acetilsalicílico, el diclofenaco y el ibuprofeno o las pirazolonas)
- Probenecid(medicamento para la gota).
- Ácidos orgánicos débiles como los diuréticosdel asa
- Medicamentos que pueden producir efectos adversos en la médula ósea, como el trimetoprim-sulfametoxazol (un antibiótico) y la pirimetamina
- Otros medicamentos utilizados para tratar la artritis reumatoidecomo la leflunomida, la sulfasalazina y la azatioprina.
- Mercaptopurina (un medicamento citostático).
- Retinoides (medicamentos para la psoriasisy otras enfermedades dermatológicas)
- Teofilina (medicamento para el asma bronquialy otras enfermedades pulmonares)
- Algunos medicamentos para las molestias estomacalescomo el omeprazol y el pantoprazol.
- Hipoglucémicos (medicamentos que se utilizan para reducir los niveles de azúcar en sangre).
Metamizol (sinónimos novaminsulfon y dipirona)(medicamento para el dolor intenso y/o la fiebre);
Las vitaminas que contienen ácido fólicopueden alterar el efecto de su tratamiento y sólo se tomarán cuando lo aconseje su médico.
Debe evitarse la vacunación con vacunas elaboradas con microorganismos atenuados.
Uso de Methofill Pencon alimentos, bebidas y alcohol
Durante el tratamiento con este medicamento, debe evitarse el consumo de alcohol, y de grandes cantidades de café, refrescos que contengan cafeína y té negro.
Embarazo, lactancia y fertilidad
Embarazo
No utilice este medicamento durante el embarazo o si está intentando quedarse embarazada. El metotrexato puede causar defectos de nacimiento, dañar al feto o provocar abortos. Se asocia a malformaciones del cráneo, la cara, el corazón y los vasos sanguíneos, el cerebro y las extremidades. Por ello, es muy importante que no se administre metotrexato a pacientes embarazadas o que tengan previsto quedarse embarazadas. En mujeres en edad fértil se debe excluir cualquier posibilidad de embarazo con las medidas oportunas, por ejemplo, una prueba de embarazo antes de empezar el tratamiento. Debe evitar quedarse embarazada mientras toma metotrexato y durante al menos 6 meses después de suspender el tratamiento, utilizando para ello métodos anticonceptivos fiables durante todo este tiempo (ver también sección «Advertencias y precauciones»).
Si se queda embarazada durante el tratamiento o sospecha que podría estar embarazada, consulte a su médico lo antes posible. Se le debe ofrecer información sobre el riesgo de efectos perjudiciales para el niño durante el tratamiento.
Si desea quedarse embarazada, consulte a su médico, quien puede derivarle a un especialista para que la informe antes del comienzo previsto del tratamiento.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Lactancia
Suspenda la lactancia materna antes y durante el tratamiento con este medicamento.
Fertilidad masculina
Los datos disponibles no indican un mayor riesgo de malformaciones ni abortos si el padre toma una dosis de metotrexato inferior a 30 mg/semana. Sin embargo, no se puede descartar por completo este riesgo. El metotrexato puede ser genotóxico, lo que significa que puede causar mutaciones genéticas. El metotrexato puede afectar a la producción de espermatozoides y causar defectos de nacimiento. Por tanto, debe evitar engendrar un hijo o donar semen mientras toma metotrexato y durante al menos 3 meses después de interrumpir el tratamiento.
Conducción y uso de máquinas
El tratamiento con este medicamento puede producir reacciones adversas que afectan al sistema nervioso central, tales como cansancio y mareos. Por lo tanto, la capacidad para conducir o utilizar máquinas puede, en ciertos casos, verse afectada. Si se encuentra cansado o somnoliento, no debe conducir ni utilizar máquinas.
Methofill Pencontiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Methofill Pen
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Su médico determinará la dosis, que será ajustada de forma individual. Normalmente el tratamiento tarda entre 4 y 8 semanas en surtir efecto.
La inyección de este medicamento se administra por vía subcutánea (debajo de la piel) por o bajo la supervisión de un médico o profesional sanitario únicamenteuna vez a la semana. Junto con su médico, usted elegirá un día de la semana que le resulte adecuado para recibir la inyección.
Al comienzo del tratamiento, Este medicamento podrá ser inyectado por el personal médico. Sin embargo, es posible que su médico decida que usted puede aprender a inyectarse Este medicamento usted mismo. Recibirá la formación adecuada para ello. En ninguna circunstancia debe intentar inyectarse usted mismo, a menos que se le haya enseñado a hacerlo.
Advertencia importante sobre la dosis de Methofill Pen(metotrexato): Use Methofill Pen solo una vez por semanapara el tratamiento de la artritis reumatoide, la artritis idiopática juvenil, la psoriasis y la artritis psoriásica y la enfermedad de Crohn. El uso excesivo de Methofill Pen (metotrexato) puede ser mortal. Lea la sección 3 de este prospecto con mucha atención. Si tiene alguna duda, consulte a su médico o farmacéutico antes de usar este medicamento. |
Uso en niños y adolescentes
El médico decide cuál es la dosis adecuada en niños y adolescentes con formas poliartríticas de artritis idiopática juvenil.
Este medicamento no está recomendado para uso en niños menores de 3 años de edad debido a que la experiencia es limitada en este grupo de edad.
Duración y forma de administración
Este medicamento se inyecta una vez a la semana.
El médico al cargo decidirá la duración del tratamiento. El tratamiento de la artritis reumatoide, artritis idiopática juvenil, psoriasis vulgaris, artritis psoriásica y enfermedad de Crohn con este medicamento es un tratamiento a largo plazo.
Al comienzo del tratamiento, este medicamento podrá ser inyectado por el personal médico. Sin embargo es posible que su médico decida que usted puede aprender a inyectarse usted mismo Methofill Pen. Recibirá la formación adecuada para ello.
En ninguna circunstancia debe intentar inyectarse usted mismo, a menos que se le haya enseñado a hacerlo.
También puede encontrar orientación sobre cómo usar este medicamento en la sección “Instrucciones de uso”.
Tenga en cuenta que hay que usar todo el contenido.
La forma de manipular y desechar el medicamento y la pluma precargada se hará conforme a la normativa local. El personal sanitario gestante no debe manipular ni administrar este medicamento.
El metotrexato no debe entrar en contacto con la superficie de la piel o las mucosas. Si entra en contacto, se debe aclarar inmediatamente el área afectada con abundante agua.
Instrucciones de uso
Recomendaciones
- Lea cuidadosamente las instrucciones antes de comenzar a administrar la inyección.
- Utilice siempre la técnica de aplicación aconsejada por su médico, enfermero o farmacéutico.
Información adicional
La forma de manipular y desechar el medicamento y la pluma precargada se hará conforme a la normativa local. El personal sanitario gestante no debe manipular ni administrar este medicamento.
El metotrexato no debe entrar en contacto con la superficie de la piel o las mucosas. Si entra en contacto, se debe aclarar inmediatamente el área afectada con abundante agua.
Lo que debe hacer antes de administrar su inyección
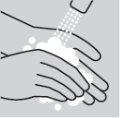
- Seleccione una superficie de trabajo limpia, plana y bien iluminada.
- Compruebe la fecha de caducidad. No usar si la fecha de caducidad ha pasado
- Coja una toallita impregnada en alcohol y un recipiente para desechar objetos punzantes
- Abra la caja que contiene la pluma precargada de metotrexato y ponga la pluma precargada de este medicamento sobre una superficie plana y limpia (como una mesa). Lea cuidadosamente el prospecto.
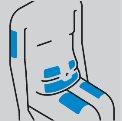
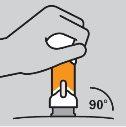
Cómo preparar la inyección
|
|
|
|
|
|
|
|
|
|
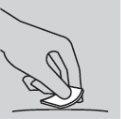
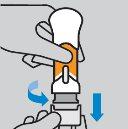
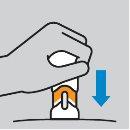
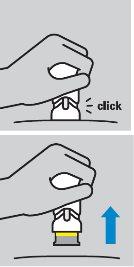
Cómo inyectarse
|
|
|
|
|
|
|
|
El metotrexato no debe entrar en contacto con la superficie de la piel o las mucosas. En caso de contaminación, deberá enjuagarse de inmediato el área afectada con abundante agua.
Con quién debe ponerse en contacto en caso de necesidad
Si tiene cualquier duda o problema, póngase en contacto con su médico, farmacéutico o enfermero.
Si usted u otra persona de su entorno se lastiman con la aguja, consulte de inmediato a su médico y no utilice esta jeringa precargada.
Eliminación y otras manipulaciones
La manipulación y la eliminación del medicamento y la jeringa precargada se realizarán de acuerdo con la normativa local. El personal sanitario gestante no deberá manipular ni administrar metotrexato.
Si usa más Methofill Pen del que debe
Si usa más medicamento del que debe, consulte a su médico inmediatamente.
Si olvidó usar Methofill Pen
No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Methofill Pen
Si interrumpe el tratamiento con este medicamento, consulte a su médico inmediatamente
Si tiene la impresión de que el efecto de este medicamento es demasiado fuerte o demasiado débil, consulte a su médico o farmacéutico.
Si sospecha que usted (o alguien más) ha administrado demasiada cantidad de Methofill Pen, contacte con su médico o acuda al hospital más cercano inmediatamente o consulte al Servicio de Información toxicológica, teléfono 91 562 04 20. Ellos decidirán qué medidas toman en función de la gravedad de la intoxicación. Lleve el medicamento consigo si acude al médico o a un hospital.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
La frecuencia y el grado de severidad de los efectos adversos dependerán de la dosis y de la frecuencia de administración. Es importante que el médico le realice controles periódicos, puesto que pueden ocurrir efectos adversos graves incluso con las dosis más bajas. Su médico le realizará pruebas para detectar anomalíasque se producen en la sangre (como recuento bajo de glóbulos blancos, recuento bajo de plaquetas o linfoma) y alteraciones en los riñones y en el hígado.
Si sufre cualquiera de los siguientes síntomas, póngase en contacto con su médico inmediatamente, ya que pueden indicar un efecto adverso grave, potencialmente mortal, que requiere un tratamiento urgente específico:
- tos seca persistente, sin expectoración, dificultad para respirar y fiebre;pueden ser signos de una inflamación de los pulmones [frecuente]
- Sangre al escupir o toser; pueden ser signos de hemorragia pulmonar [frecuencia no conocida]
- síntomas de daño hepático, como amarillamiento de la piel o del blanco de los ojos; metotrexato puede provocar lesión crónica del hígado (cirrosis hepática), formación de tejido cicatricial en el hígado (fibrosis hepática), degeneración grasa del hígado [todas poco frecuentes], inflamación del hígado (hepatitis aguda) [rara] e insuficiencia hepática [muy rara]
- síntomas alérgicos, como erupción cutánea incluidos picazón en la piel, hinchazón de las manos, pies, tobillos, cara, labios, boca o garganta (lo que puede provocar dificultad para tragar o respirar) y sensación de desmayo;estos pueden ser signos de reacciones alérgicas graves o de shock anafiláctico [raros]
- síntomas de daño renal, como hinchazón de las manos, tobillos o pies o cambios en la frecuencia de la micción o disminución (oliguria) o ausencia (anuria) de orina;estos pueden ser signos de insuficiencia renal [raros]
- síntomas de infecciones p.ej., fiebre, escalofríos, dolores, dolor de garganta;metotrexato puede hacerle más propenso a las infecciones. Pueden producirse infecciones graves como un tipo determinado de neumonía (neumonía por Pneumocystis jirovecii) o septicemia (sepsis) [raras]
- síntomas tales como debilidad en un lado del cuerpo (ictus) o dolor, hinchazón, enrojecimiento y calor inusual en una de las piernas (trombosis venosa profunda); esto puede suceder cuando un coágulo sanguíneo desprendido causa una obstrucción de un vaso sanguíneo(evento tromboembólico) [raros]
- fiebre y deterioro grave de su estado general, o fiebre repentina acompañada de dolor de garganta o dolor de boca, o problemas urinarios; el metotrexato puede producir una caída brusca del número de ciertos glóbulos blancos (agranulocitosis) y mielosupresión grave [muy raras].
- hemorragia inesperada, p.ej., encías sangrantes, sangre en la orina, vómitos con sangre o hematomas;estos pueden ser signos de una disminución severa del número de plaquetas causada por episodios graves de depresión de la médula ósea [muy raros]
- síntomas tales como dolor de cabeza intenso a menudo en combinación con fiebre, rigidez del cuello, náuseas, vómitos, desorientación y sensibilidad a la luzpueden indicar una inflamación de las membranas del cerebro (meningitis aséptica aguda) [muy rara]
- se han notificado ciertos trastornos del cerebro (encefalopatía/leucoencefalopatía) en pacientes con cáncer tratados con metotrexato; estos efectos adversos no pueden descartarse cuando el tratamiento con metotrexato se utiliza para tratar otras enfermedades; los signos de este tipo de trastornos del cerebro pueden ser alteración del estado mental, trastornos del movimiento (ataxia), trastornos visuales o trastornos de la memoria[frecuencia no conocida]
- erupción grave de la piel o formación de ampollas en la piel (esto también puede afectar a la boca, los ojos y los genitales); estos pueden ser signos de una afección llamada síndrome de Stevens Johnson o síndrome de la piel escaldada (necrólisis epidérmica tóxica/síndrome de Lyell) [muy raros]
A continuación, encontrará los demás efectos adversos que pueden ocurrir:
Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- Inflamación del revestimiento de la boca, indigestión, náuseas, pérdida del apetito, dolor abdominal.
- Resultados anómalos en las pruebas de la función hepática (ASAT, ALAT, bilirrubina, fosfatasa alcalina.
Frecuentes:pueden afectar hasta 1 de cada 10 personas
- Úlceras bucales, diarrea.
- Erupción, enrojecimiento de la piel, picor.
- Dolor de cabeza, cansancio, somnolencia.
- Disminución de la formación de células sanguíneas con disminución en el número de glóbulos blancos, rojos o plaquetas
Poco frecuentes:pueden afectar hasta 1 de cada 100 personas
- Inflamación de la garganta.
- Inflamación del intestino, vómitos, inflamación del páncreas, heces negras o alquitranosas, úlceras gastrointestinales y hemorragia.
- Reacciones similares a quemaduras solares debido a una mayor sensibilidad de la piel a la luz solar, caída del pelo, aumento del número de nódulos reumáticos, úlcera cutánea, herpes zóster, inflamación de los vasos sanguíneos, erupción tipo herpes, urticaria.
- Aparición de diabetes mellitus.
- Mareos, confusión, depresión.
- Disminución de la albúmina sérica.
- Disminución del número de todas las células sanguíneas y plaquetas.
- Inflamación y úlcera de la vejiga urinaria o vagina, disminución de la función renal, trastornos urinarios.
- Dolor en las articulaciones, dolor muscular, reducción de la masa ósea.
Raros:pueden afectar hasta 1 de cada 1.000 personas
- Inflamación del tejido de las encías.
- Aumento de la pigmentación de la piel, acné, moratones en la piel debidos a la hemorragia de los vasos(equimosis, petequias), inflamación alérgica de los vasos sanguíneos.
- Disminución del número de anticuerpos en la sangre.
- Infección (incluida la reactivación de infecciones crónicas inactivas), ojos rojos (conjuntivitis).
- Cambios del estado de ánimo (alteraciones del estado de ánimo).
- Trastornos visuales.
- Inflamación del saco alrededor del corazón, acumulación de líquido en el saco alrededor del corazón, obstrucción del llenado cardíaco a causa de la presencia de líquido en el saco que rodea el corazón.
- Tensión arterial baja.
- Formación de tejido cicatricial en el pulmón (fibrosis pulmonar), dificultad respiratoria y asma bronquial, acumulación de líquido en el saco que rodea el pulmón.
- Fractura por estrés.
- Alteraciones de los electrolitos.
- Fiebre, alteraciones en la curación de las heridas.
Muy raros:pueden afectar hasta 1 de cada 10.000 personas
- Dilatación tóxica y aguda del intestino (megacolon tóxico).
- Aumento de la pigmentación de las uñas, inflamación de las cutículas (paroniquia aguda), infección profunda de los folículos del pelo (furunculosis), agrandamiento visible de los vasos sanguíneos pequeños.
- Dolor, pérdida de fuerza o sensación de adormecimiento u hormigueo/sensibilidad a los estímulos menor de la normal, alteraciones del gusto (sabor metálico), convulsiones, parálisis, meningismo.
- Alteración de la visión, trastorno no inflamatorio de los ojos (retinopatía).
- Pérdida del apetito sexual, impotencia, aumento de las mamas masculinas, formación alterada del esperma (oligospermia), trastornos menstruales, secreción vaginal.
- Aumento del tamaño de los nódulos linfáticos (linfoma).
- Trastornos linfoproliferativos (aumento excesivo de glóbulos blancos).
Frecuencia no conocida:no puede estimarse a partir de los datos disponibles
- Aumento del número de ciertos glóbulos blancos.
- Hemorragia nasal.
- Proteínas en la orina.
- Sensación de debilidad.
- Lesión en los huesos de la mandíbula (secundaria a un aumento excesivo de glóbulos blancos).
- Destrucción del tejido en el lugar de la inyección.
- Enrojecimiento y descamación de la piel.
- Hinchazón.
La administración subcutánea de metotrexato se tolera bien a nivel local. Únicamente se observaron reacciones cutáneas locales leves (como sensaciones de quemazón, eritema, hinchazón, cambio de color, picor severo, dolor), que disminuyeron durante el tratamiento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: a www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Methofill Pen
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar por debajo de 30 ºC.
Conservar las plumas precargadas en el embalaje exterior para protegerlas de la luz.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la pluma precargada después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Methofill Pen
- El principio activo es metotrexato. 1 ml de solución contiene metotrexato disódico que corresponde a 50 mg de metotrexato.
1 pluma precargada con 0,40 ml de solución contiene 20 mg de metotrexato
- Los demás componentes son cloruro de sodio, hidróxido de sodio para ajustar el pH, agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Las plumas precargadas de Methofill Pen contienen una solución amarilla-marrón transparente. Se comercializan los siguientes tamaños de envases:
Jeringas precargadas que contienen 0,15 ml, 0,20 ml, 0,25 ml, 0,30 ml, 0,35 ml, 0,40 ml, 0,45 ml, 0,50 ml, 0,55 ml y 0,60 ml de solución inyectable disponibles en envases de 1 o multipacks de 4 (4 packs de 1) plumas precargadas.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Accord Healthcare S.L.U.
World Trade Center
Moll de Barcelona, s/n
Edifici Est 6ª planta,
08039 Barcelona,
España
Responsable de la fabricación:
Accord Healthcare Polska Sp.z.o.o.
ul.Lutomierska 50
pabianice, 95-200
Polonia
ó
Laboratori Fundació DAU
C/ C, 12-14 Pol. Ind. Zona Franca, Barcelona
España
Representante Local:
Laboratorios Rubió, S.A.
Industria 29
Polígono Industria Comte de Sert
08755 Castellbisbal
(Barcelona)
España
Fecha de la última revisión de este prospecto: Agosto 2024
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.es
- País de registro
- Precio medio en farmacia106.47 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a METHOFILL PEN 20 MG/0,40 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA EFGForma farmacéutica: INYECTABLE, 10 mg/ 1 mlPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere recetaForma farmacéutica: INYECTABLE, 15 mgPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere recetaForma farmacéutica: INYECTABLE, 20 mgPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere receta
Médicos online para METHOFILL PEN 20 MG/0,40 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de METHOFILL PEN 20 MG/0,40 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes