
MERIOFERT KIT 150 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar MERIOFERT KIT 150 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Meriofert Kit 150 UIpolvo y disolvente para solución inyectable
menotropina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- En este prospecto, Meriofert 150 UI de polvo y disolvente para solución inyectable se denomina Meriofert Kit.
Contenido del prospecto
- Qué es Meriofert Kit y para qué se utiliza
- Qué necesita saber antes de empezar a usar Meriofert Kit
- Cómo usar Meriofert Kit
- Posibles efectos adversos
- Conservación de Meriofert Kit
- Contenido del envase e información adicional
1. Qué es Meriofert Kit y para qué se utiliza
- Meriofert Kit se usa para estimular la ovulación en mujeres que no ovulan y que no han
respondido a otro tratamiento (citrato de clomifeno).
- Meriofert Kit se usa para provocar el desarrollo de varios folículos (y, por tanto, de varios
óvulos) en mujeres sometidas a un tratamiento de fertilidad.
Meriofert Kit es una gonadotropina menopáusica humana muy purificada, que pertenece a un grupo de medicamentos denominados gonadotropinas.
Cada vial contiene polvo liofilizado con 150 UI de actividad de hormona foliculoestimulante (FSH) humana y 150 UI de actividad de hormona luteinizante humana (LH).
La gonadotropina menopáusica humana (HMG) es extraída de la orina de mujeres post menopáusicas. Se añade gonadotropina coriónica humana (hCG), extraída de la orina de mujeres embarazadas, para contribuir a la actividad de LH total.
Este medicamento debe usarse bajo la supervisión de un médico.
2. Qué necesita saber antes de empezar a usar Meriofert Kit
Antes de comenzar el tratamiento, se evaluará su fertilidad y la de su pareja.
No use Meriofert Kit
- Si presenta un aumento del tamaño de sus ovarios, o quistes cuya causa no sea un trastorno hormonal (enfermedad ovárica poliquística).
- Si tiene sangrados de origen desconocido.
- Si padece cáncer de ovarios, útero o mama.
- Si presenta una hinchazón anormal (tumor) de la hipófisis o el hipotálamo (cerebro).
- Si es alérgica a la menotropina o a cualquiera de los demás componentes de este
medicamento (incluidos en la sección 6).
No debe utilizar este medicamento si sufre menopausia precoz, malformación de los órganos genitales o determinados tumores del útero que impedirían un embarazo normal.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Meriofert Kit.
Aunque no se han descrito todavía reacciones alérgicas a Meriofert Kit, deberá informar a su médico si padece alguna reacción alérgica a medicamentos similares.
Este tratamiento aumenta el riesgo de padecer una enfermedad denominada síndrome de hiperestimulación ovárica (SHO)(ver sección 4). Si se produce una hiperestimulación ovárica, se suspenderá el tratamiento y deberán evitarse los embarazos. Los primeros signos de hiperestimulación ovárica son dolor en la región inferior del abdomen, náuseas (malestar), vómitos y ganancia de peso. Si se producen dichos síntomas, deberá examinarle un médico lo antes posible. En casos graves, pero raros, pueden agrandarse los ovarios y acumularse líquido en el abdomen o en el pecho.
El medicamento empleado para provocar el desprendimiento definitivo de los óvulos maduros (que contiene gonadotropina coriónica humana, hCG) puede aumentar la probabilidad de sufrir un SHO. Por tanto, no es recomendable utilizar hCG en los casos en los que se esté produciendo un SHO y no deberán mantenerse relaciones sexuales durante un mínimo de 4 días, aunque se utilice un método anticonceptivo de barrera.
Cabe destacar que las mujeres con problemas de fertilidad tienen una tasa de abortos superior a la de la población normal.
La frecuencia de embarazos y partos múltiples en las pacientes sometidas a un tratamiento para estimular la ovulación es mayor que en las mujeres que conciben de forma natural. Sin embargo, este riesgo puede reducirse al mínimo si se usa la dosis recomendada.
El riesgo de embarazo extrauterino (embarazo ectópico) es ligeramente mayor en las mujeres con lesiones en las trompas de Falopio.
Los embarazos múltiples y las características de los progenitores sometidos a tratamientos de fertilidad (por ejemplo, la edad de la madre o las características del semen) pueden estar asociados a un aumento del riesgo de defectos de nacimiento.
El tratamiento con Meriofert Kit, al igual que el propio embarazo, puede aumentar la probabilidad de sufrir una trombosis. La trombosis es la formación de un coágulo de sangre en un vaso sanguíneo, la mayoría de las veces en las venas de las piernas o los pulmones.
Comente este hecho con su médico antes de empezar el tratamiento, en especial:
- Si ya sabe que tiene una mayor probabilidad de sufrir una trombosis.
- Si usted o algún familiar cercano ha sufrido alguna vez una trombosis.
- Si padece sobrepeso grave.
Población pediátrica
El medicamento no está indicado para uso pediátrico.
Uso deMeriofert Kit con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
La influencia de Meriofert Kit sobre la capacidad para conducir y utilizar máquinas es nula o insignificante..
Meriofert Kit contiene sodio
Este medicamento contiene menos de 23 mg (1 mmol) de sodio, por lo que se considera esencialmente “exento de sodio”
3. Cómo usar Meriofert Kit
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Dosis y duración del tratamiento
Mujeres que no ovulan y tienen periodos irregulares o ausencia de menstruación
Por norma general, la primera inyección de un vial de 75 UI de menotropina se administra durante la primera semana del ciclo tras la menstruación espontánea o inducida.
Posteriormente, se inyecta todos los días la dosis de este medicamento recetada por el médico y se continúa con el tratamiento hasta que se hayan formado uno o más folículos maduros en el ovario. El médico le ajustará la dosis de este medicamento dependiendo de la respuesta ovárica, que se determina por medio de exploraciones clínicas.
En cuanto un folículo alcance la fase de desarrollo necesaria, se interrumpirá el tratamiento con este medicamento y se provocará la ovulación con otra hormona (gonadotropina coriónica, hCG).
La ovulación se produce, por lo general, en un plazo de entre 32 y 48 horas.
En esta fase del tratamiento existe la posibilidad de fertilización. Se le recomendará que mantenga relaciones sexuales todos los días a partir del día anterior a la administración de hCG. Si a pesar de la ovulación no se consigue un embarazo, puede repetirse el tratamiento.
Mujeres sometidas a una estimulación ovárica para el desarrollo folicular múltiple antes de una fertilizaciónin vitrou otras técnicas de reproducción asistida
La finalidad de este método es obtener un desarrollo folicular múltiple simultáneo. El tratamiento comenzará el segundo o tercer día del ciclo con inyecciones de entre 150 y 300 UI de este medicamento (1 ó 2 viales de Meriofert Kit 150 UI). El médico puede optar por administrarle dosis más elevadas si es necesario. La dosis de este medicamento que se inyecta es mayor que la del método empleado para la fertilización natural. El médico se encarga de ajustar la continuación del tratamiento de forma individualizada.
En cuanto se ha desarrollado un número suficiente de folículos, se interrumpe el tratamiento con este medicamento y se provoca la ovulación mediante la inyección de otra hormona (gonadotropina coriónica, hCG).
Cómo administrar Meriofert Kit
Este medicamento se administra en forma de inyección bajo la piel (por vía subcutánea) o en el músculo (por vía intramuscular).
Los viales sólo deben usarse una vez y la inyección debe administrarse en cuanto esté preparada.
Después de aconsejarle e instruirle debidamente, es posible que el médico le pida que se administre la inyección de Meriofert Kit usted misma.
La primera vez que lo haga, el médico debe
- permitirle que practique la autoadministración de una inyección subcutánea,
- indicarle los lugares en los que puede administrarse la inyección,
- enseñarle a preparar la solución inyectable,
- explicarle cómo preparar la dosis correcta para la inyección.
Antes de administrarse la inyección de Meriofert Kit, lea detenidamente las siguientes instrucciones.
Cómo preparar e inyectar 1 vial de Meriofert Kit
La inyección debe prepararse justo antes de que usted esté lista para usarla, empleando para ello la jeringa precargada de disolvente (una solución de cloruro de sodio de 9 mg/ml en agua para inyectables) incluida en cada envase de Meriofert Kit.
Prepare una superficie limpia y lávese las manos. Es importante que las manos y los artículos que utilice estén lo más limpios posible.
Coloque sobre la superficie los artículos siguientes:
- dos algodones con alcohol (no incluidos),
- un vial con polvo de Meriofert Kit,
- una jeringa precargada con disolvente,
- una aguja para preparar la inyección,
- una aguja fina para la inyección subcutánea.
RECUERDE: no retire la lengüeta de apoyo (pieza blanca) de la jeringa precargada, ya que evita la extracción involuntaria del pistón y mejora el manejo de la jeringa durante la inyección.
Reconstitución de la solución inyectable
Preparación de la inyección
- • Retire el capuchón de la jeringa precargada; introduzca la aguja de
reconstitución (aguja larga) en la jeringa.
•Coloque con cuidado la jeringa sobre la superficie limpia.
•Evite tocar la aguja.
Prepare la solución para la inyección

- • Retire la cápsula de plástico coloreada (verde claro en la presentación
de 75 UI y verde oscuro en la de 150 UI) del vial de Meriofert Kit
empujándola suavemente hacia arriba.
- Limpie la parte superior de goma con el algodón impregnado de
alcohol y espere a que se seque.
- • Coja la jeringa, retire el capuchón protector de la aguja e introduzca la
aguja a través del centro de goma de la parte superior del vial de
Meriofert Kit.
- Empuje con firmeza el émbolo hacia abajo para rociar toda la
solución sobre el polvo.
- NO AGITE EL VIAL; muévalo suavemente en círculos hasta
obtener una solución transparente.
Por lo general, el medicamento se disuelve inmediatamente.
- • Con la aguja todavía introducida, dele la vuelta al vial.
- Asegúrese de que la punta de la aguja esté por debajo del nivel del
líquido.
- Tire con cuidado del émbolo para introducir toda la solución de
Meriofert Kit en la jeringa.
•Compruebe que la solución reconstituida sea trasparente.
Cuando reconstituya más de 1 vial de Meriofert Kit, introduzca de nuevo el contenido del primer vial en la jeringa e inyéctelo lentamente en un segundo vial después de repetir los pasos del 2 al 4.
Inyección del medicamento por vía subcutánea
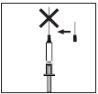
 Cuando la jeringa contenga la dosis descrita, coloque el capuchón protector de la aguja. Retire la aguja de la jeringa y sustitúyala por la aguja fina para inyección subcutánea con su capuchón protector.
Cuando la jeringa contenga la dosis descrita, coloque el capuchón protector de la aguja. Retire la aguja de la jeringa y sustitúyala por la aguja fina para inyección subcutánea con su capuchón protector.- Introduzca con firmeza la aguja fina en el cilindro de la jeringa y, a continuación, gírela ligeramente para asegurarse de que está totalmente enroscada y conseguir una conexión estanca.
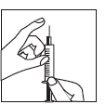
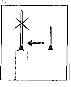
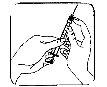 Retire el capuchón protector de la aguja. Sujete la jeringa con la aguja apuntando hacia arriba y dé golpecitos suaves en el lateral de la jeringa para que las burbujas de aire suban hasta la parte superior;
Retire el capuchón protector de la aguja. Sujete la jeringa con la aguja apuntando hacia arriba y dé golpecitos suaves en el lateral de la jeringa para que las burbujas de aire suban hasta la parte superior;- Empuje el émbolo hasta que aparezca una gota de líquido en la punta de la aguja.
- No lo utilice si contiene partículas o está turbio.
Zona de inyección
- El médico o el enfermero ya le habrá explicado en qué parte del cuerpo debe administrarse el medicamento. Los lugares habituales son el muslo o la pared inferior del abdomen bajo el ombligo.
- Limpie la zona de inyección con un algodón con alcohol.
Inserción de la aguja
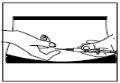
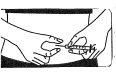
? Pellizque y apriete con firmeza la piel. Con la otra mano, introduzca
la aguja con un movimiento seco y rápido, formando un ángulo de
45° o 90°.
Inyección de la solución
- Inyecte la jeringa bajo la piel tal como le indicaron. No la inyecte directamente en una vena. Empuje el émbolo lentamente y sin interrupciones, para que la solución se inyecte correctamente y los tejidos cutáneos no sufran daños.
Tómese el tiempo que necesite para inyectar el volumen de solución que le han recetado. Dependiendo de la dosis que le recete su médico, es posible que no utilice todo el volumen de la solución.
Extracción de la aguja
- Extraiga rápidamente la jeringa y presione la zona de inyección con un algodón con desinfectante. Los masajes suaves en la zona (mientras se mantiene la presión) ayudan a dispersar la solución de Meriofert Kit y alivian las posibles molestias.
Inyección del medicamento por vía intramuscular
En el caso de las inyecciones intramusculares, el profesional sanitario preparará e inyectará este medicamento en el lateral del muslo o el glúteo.
Deseche todos los artículos utilizados
Una vez finalizada la inyección, introduzca todas las agujas, los viales y jeringas vacíos en el contenedor para objetos punzocortantes. La eliminación de la solución no utilizada y de todos los materiales que hayan estado en contacto con ella, se realizará de acuerdo con la normativa local.
Si usa más MeriofertKitdel que debe
Se desconocen los efectos de la sobredosis de este medicamento, no obstante, cabría esperar que se produjera un síndrome de hiperestimulación ovárica (ver sección 4). Si usa más medicamento del que debe, consulte a su médico o enfermero.
Si olvidó usar Meriofert Kit
Úselo en el plazo en que le tocaría normalmente la siguiente inyección. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Meriofert Kit
No lo interrumpa por iniciativa propia.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
El siguiente efecto adverso es importante y exigirá una intervención inmediata si lo padece. Deberá dejar de tomar este medicamento y acudir al médico de inmediato si se produce lo siguiente:
Frecuentes, puede afectar hasta 1 de cada 10 personas
- Síndrome de hiperestimulación ovárica (los síntomas comprenden la formación de quistes ováricos o el aumento del tamaño de quistes existentes, dolor en la parte inferior del estómago, sensación de sed y náuseas con vómitos ocasionales, evacuación de pequeñas cantidades de orina concentrada y ganancia de peso) (ver sección 2 para obtener información adicional).
También se han comunicado los siguientes efectos adversos:
Muy frecuentes: pueden afectar a más de 1 de cada 10 personas
- Cefalea
- Hinchazón del estómago o meteorismo
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- Dolor o molestia abdominal
- Dolor pélvico
- Dolor de espalda
- Sensación de pesadez
- Molestia en mama
- Mareo
- Sofocos
- Sed
- Sensación de enfermedad
- Cansancio
- Malestar general
- Reacciones en la zona de inyección, tales como dolor e inflamación (más habituales con la inyección IM que con la SC).
Raras: pueden afectar hasta 1 de cada 1,000 personas
- Torsión ovárica (rotación del ovario que causa un dolor de gran intensidad en la parte inferior del abdomen)
- Tromboembolia (formación de un coágulo en un vaso sanguíneo que se desprende y viaja a través del torrente sanguíneo para bloquear otro vaso).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Meriofert Kit
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25°C. Conservar el vial y la jeringa precargada de disolvente en el embalaje exterior para protegerlos de la luz.
No utilice este medicamento después de la fecha de caducidad que aparece en el embalaje exterior, el vial y la jeringa precargada de disolvente después de CAD. Si la fecha de caducidad se indica como mes/año, la fecha de caducidad es el último día del mes que se indica.
Utilizar inmediatamente después de la reconstitución.
No utilice este medicamento si observa que la solución no es transparente. Tras la reconstitución, la solución debe ser transparente e incolora.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE  de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Meriofert Kit
El principio activo es menotropina.
Cada vial contiene polvo liofilizado con 150 UI de actividad de hormona foliculoestimulante (FSH) humana y 150 UI de actividad de hormona luteinizante humana (LH).
La gonadotropina menopáusica humana (HMG) es extraída de la orina de mujeres post menopáusicas.
Se añade gonadotropina coriónica humana (hCG), una hormona extraída de la orina de las mujeres embarazadas, para contribuir a la actividad de LH total.
Si se utilizan varios viales de polvo, la cantidad de menotropina en 1 ml de solución reconstituida será la siguiente:
Meriofert Kit 150UI polvo y disolvente para solución inyectable | |
Número de viales utilizados | Cantidad total de menotropina en 1 ml de solución |
1 | 150 UI |
2 | 300 UI |
3 | 450 UI |
Los demás componentes son
Vial de polvo: lactosa monohidrato
Jeringa precargada con disolvente: solución de 9 mg/ml de cloruro sódico.
Aspecto del producto y contenido del envase
Polvo: torta o polvo blanco liofilizado
Disolvente: solución transparente e incolora
Meriofert Kit se presenta como polvo y disolvente para solución inyectable.
1 estuche contiene lo siguiente:
- Un vial con torta o polvo blanco liofilizado
- Una jeringa precargada (1 ml) con solución transparente e incolora
- Una aguja para la reconstitución y la inyección intramuscular (aguja larga)
- Una aguja para la inyección subcutánea (aguja corta)
Se suministra en cajas de 1, 5 ó 10 estuches.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de fabricación
Titular de la autorización de comercialización
IBSA Farmaceutici Italia Srl
Via Martiri di Cefalonia 2
26900 Lodi, Italia
Responsable de la fabricación
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia, 2
26900 Lodi – Italia
o (para Reino Unido)
IBSA Pharma Limited
Units 4-6
Colonial Business Park
Colonial Way
Watford D24 4PR
Reino Unido
Puede solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Instituto Bioquimico Iberico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (España)
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres (las concentraciones y formas farmacéuticas son idénticas en todos los países, sólo cambian los nombres comerciales):
Austria: Meriofert PFS
Bélgica: Fertinorm Kit
Bulgaria: Meriofert PFS
Chipre: Meriofert PFS
República Checa: Meriofert Set
Dinamarca: Meriofert Set
Estonia: Meriofert Set
Finlandia: Meriofert Set
Francia: Fertistartkit
Grecia: Meriofert
Hungria: Meriofert Kit
Italia: Meriofert
Letonia: Meriofert Set
Lituania: Meriofert Set
Luxemburgo: Fertinorm Kit
Noruega: Meriofert Set
Polonia: Mensinorm Set
Rumania: Meriofert PFS
Eslovaquia: Meriofert Kit
España: Meriofert Kit
Países Bajos: Meriofert spuit
Reino Unido: Meriofert PFS
Suecia: Meriofert Set
Fecha de la última revisión de este prospecto: Mayo 2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MERIOFERT KIT 150 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, -Principio activo: human menopausal gonadotrophinFabricante: Angelini Pharma Espana S.L.Requiere recetaForma farmacéutica: INYECTABLE, 1200 UIPrincipio activo: human menopausal gonadotrophinFabricante: Ferring S.A.Requiere recetaForma farmacéutica: INYECTABLE, 1.200 UIPrincipio activo: human menopausal gonadotrophinFabricante: Ferring S.A.U.Requiere receta
Médicos online para MERIOFERT KIT 150 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MERIOFERT KIT 150 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






