
LUMIGAN 0,1 mg/ml COLIRIO EN SOLUCION

Cómo usar LUMIGAN 0,1 mg/ml COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
LUMIGAN 0,1mg/ml, colirio en solución
bimatoprost
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es LUMIGAN 0,1 mg/ml y para qué se utiliza
- Qué necesita saber antes de empezar a usar LUMIGAN 0,1 mg/ml
- Cómo usar LUMIGAN 0,1 mg/ml
- Posibles efectos adversos
- Conservación de LUMIGAN 0,1 mg/ml
- Contenido del envase e información adicional
1. Qué es LUMIGAN 0,1 mg/ml y para qué se utiliza
LUMIGAN es un medicamento para el glaucoma. LUMIGAN pertenece a un grupo de medicamentos llamados prostamidas.
LUMIGAN colirio se utiliza para reducir la presión elevada del ojo. LUMIGAN se puede usar solo o con otros colirios llamados betabloqueantes que también reducen la presión.
El ojo contiene un líquido transparente, acuoso, que mantiene la parte interior del ojo. Este líquido se drena continuamente fuera del ojo y se genera nuevo líquido para reemplazarlo. Si el líquido no se drena con la suficiente velocidad, aumenta la presión dentro del ojo. LUMIGAN actúa aumentando el drenaje del líquido. Esto reduce la presión dentro del ojo. Si esta presión no se reduce, podría provocar una enfermedad denominada glaucoma y dañar su visión.
2. Qué necesita saber antes de empezar a usar LUMIGAN 0,1 mg/ml
No use LUMIGAN 0,1 mg/ml :
- si es alérgico al bimatoprost o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tuvo que interrumpir la utilización de las gotas oculares en el pasado debido a un efecto secundario del conservante cloruro de benzalconio.
Advertencias y precauciones:
Consulte a su médico o farmacéutico antes de empezar a usar LUMIGAN 0,1 mg/ml
- Hable con su médico si:
- tiene algún problema respiratorio.
- tiene problemas de hígado o riñones.
- ha tenido una cirugía de catarata en el pasado.
- padece ojo seco
- tiene o ha tenido algún problema de la córnea (parte frontal transparente del ojo)
- usa lentes de contacto (consulte “LUMIGAN 0,1 mg/ml contiene cloruro de benzalconio”)
- presenta o ha presentado una tensión arterial baja o una frecuencia cardiaca baja
- ha padecido una infección viral o una inflamación del ojo
Durante el tratamiento, LUMIGAN puede provocar una pérdida de grasa alrededor del ojo que puede causar profundización del surco palpebral, hundimiento de los ojos (enoftalmos), caída de los párpados superiores (ptosis), estiramiento de la piel alrededor del ojo (involución de la dermatocalasis) y que la parte blanca inferior del ojo se haga más visible (exposición escleral inferior). Los cambios suelen ser leves, pero si se acentúan, pueden afectar a su campo de visión. Los cambios pueden desaparecer si deja de usar LUMIGAN. LUMIGAN también puede causar oscurecimiento y crecimiento de las pestañas, así como oscurecimiento de la piel alrededor del párpado. Puede oscurecerse el color del iris. Estos cambios puede que sean permanentes y más visibles si sólo se está tratando un ojo.
Niños y adolescentes
LUMIGAN no se ha estudiado en menores de 18 años y, por tanto, no debe utilizarse en pacientes de menos de 18 años de edad.
Otros medicamentos y LUMIGAN:
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
LUMIGAN puede pasar a la leche materna, por tanto no debería utilizarlo si está en período de lactancia.
Conducción y uso de máquinas:
Tras la instilación de LUMIGAN, puede aparecer visión borrosa durante un corto período de tiempo. No conduzca ni use máquinas hasta ver con claridad.
LUMIGAN 0,1 mg/ml contiene cloruro de benzalconio
Este medicamento contiene 0,6 mg de cloruro de benzalconio en cada 3 ml de solución, lo que equivale a 0,2 mg/ml.
No utilice el colirio cuando lleve puestas sus lentes. El cloruro de benzalconio, conservante de Lumigan, se puede absorber por las lentes de contacto blandas y puede alterar el color de las lentes de contacto. Retirar las lentes de contacto antes de usar este medicamento y esperar 15 minutos antes de volver a colocarlas. El cloruro de benzalconio puede causar irritación ocular, especialmente si padece de ojo seco u otras enfermedades de la cornea (capa transparente de la zona frontal del ojo). Consulte a su medico si siente una sensacion extrana, escozor o dolor en el ojo despues de usar este medicamento.
3. Cómo usar LUMIGAN 0,1 mg/ml
Siga exactamente las instrucciones de administración del medicamento indicadas por su médico o farmacéutico. En caso de duda, pregunte a su médico o farmacéutico.
LUMIGAN debe ser utilizado únicamente en el ojo. La dosis recomendada es una gota de LUMIGAN en cada ojo que precise tratamiento, una vez al día, a última hora de la tarde.
Si utiliza LUMIGAN con otra medicación ocular, espere al menos cinco minutos entre el uso de LUMIGAN y de la otra medicación ocular
No utilice el medicamento más de una vez al día, ya que puede reducirse la efectividad del tratamiento.
Instrucciones de uso:
No debe usar el envase si el sello de protección está roto antes de utilizarlo por primera vez.
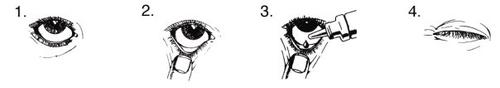
- Lávese las manos. Incline la cabeza hacia atrás y mire hacia el techo.
- Tire suavemente hacia abajo del párpado inferior, hasta que haga un pequeño hueco.
- Invierta el envase y apriételo para que caiga una gota en cada ojo que precise tratamiento.
- Suelte el párpado inferior y mantenga el ojo cerrado durante 30 segundos.
Limpie cualquier exceso que corra por su mejilla.
Si la gota cae fuera del ojo, vuélvalo a intentar.
Para ayudar a prevenir las infecciones y evitar las lesiones oculares, impida que la punta del envase toque el ojo ni ninguna otra superficie. Vuelva a poner el tapón y cierre el envase inmediatamente después de usarlo.
Si usa másLUMIGAN 0,1 mg/mldel que debe
Si usa más LUMIGAN del que debe, es improbable que ello le cause ningún daño serio. Aplique la siguiente dosis a la hora habitual. Si está preocupado/a, hable con su médico o farmacéutico.
Si olvidó usarLUMIGAN 0,1 mg/ml
Si olvidó aplicar LUMIGAN, use una sola gota tan pronto como se acuerde y vuelva después a su rutina habitual. No aplique una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento con LUMIGAN 0,1 mg/ml
LUMIGAN debe usarse cada día para que funcione bien. Si deja de usar LUMIGAN, la presión en el interior del ojo puede aumentar, así pues, consulte con su médico antes de interrumpir el tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos muy frecuentes
Éstos pueden afectar a 1 o más pacientes de cada 10
Que afectan al ojo
- Ligero enrojecimiento (hasta el 29% de las personas)
- Pérdida de grasa en la región del ojo que puede causar profundización del surco palpebral, hundimiento de los ojos (enoftalmos), caída de los párpados (ptosis), estiramiento de la piel alrededor del ojo (involución de la dermatocalasis) y que la parte blanca inferior del ojo se haga más visible (exposición escleral inferior).
Efectos adversos frecuentes
Estos pueden afectar a entre 1 y 9 pacientes de cada 100
Que afectan al ojo
- Pequeñas erosiones epiteliales en el ojo, con o sin inflamación
- Irritación
- Picazón en los ojos
- Pestañas más largas
- Irritación al instilar la gota en el ojo
- Dolor ocular
Que afectan a la piel
- Párpados enrojecidos y con picor
- Piel de color más oscuro alrededor del ojo.
- Crecimiento del pelo alrededor del ojo.
Efectos adversos poco frecuentes
Éstos pueden afectar a entre 1 y 9 pacientes de cada 1000
Que afectan al ojo
- Color más oscuro del iris
- Cansancio ocular
- Inflamación de la superficie del ojo
- Visión borrosa
- Pérdida de las pestañas
Que afectan a la piel
- Sequedad cutánea
- Descamación del reborde palpebral
- Hinchazón del párpado
- Picor
Que afectan al cuerpo
- Dolor de cabeza
- Sensación de malestar
Efectos adversos de frecuencia no conocida
Que afectan al ojo
- Edema macular (inflamación de la retina en la parte posterior del ojo que puede dar lugar a un empeoramiento de la visión)
- Párpado más oscuro
- Sequedad
- Ojos pegajosos
- Sensación de cuerpo extraño en el ojo
- Inflamación del ojo
- Aumento de las lágrimas
- Molestias oculares
- Sensibilidad a la luz
Que afectan al cuerpo
- Asma
- Empeoramiento del asma
- Empeoramiento de la enfermedad pulmonar denominada enfermedad pulmonar obstructiva crónica (EPOC)
- Dificultad para respirar
- Síntomas de reacción alérgica (inflamación, enrojecimiento del ojo y erupción en la piel)
- Mareo
- Presión arterial elevada
- Decoloración de la piel (periocular)
Además de los efectos adversos de LUMIGAN 0,1 mg/ml, se han observado los siguientes efectos adversos con otro medicamento que contiene una mayor concentración de bimatoprost (0,3 mg/ml):
- Quemazón ocular
- Una reacción alérgica en el ojo
- Párpados inflamados
- Dificultad para ver con claridad
- Empeoramiento de la visión
- Inflamación de la capa transparente que cubre el ojo
- Lágrimas
- Pestañas más oscuras
- Hemorragia retiniana
- Inflamación en el interior del ojo
- Edema macular cistoide (inflamación de la retina en el interior del ojo que da lugar a un empeoramiento de la visión)
- Fasciculaciones del párpado
- El párpado se ha contraído y separado de la superficie del ojo
- Enrojecimiento cutáneo alrededor del ojo
- Debilidad
- Un aumento en los resultados de los análisis de sangre que muestran actividad hepática
Otros efectos adversos notificados con colirios que contienen fosfato
En muy raras ocasiones, algunos pacientes con lesiones graves de la capa transparente situada en la parte delantera del ojo (la córnea) han desarrollado manchas turbias en la córnea debidas a la acumulación de calcio durante el tratamiento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de los efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de LUMIGAN 0,1 mg/ml
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del envase y en la caja después de EXP. La fecha de caducidad es el último día del mes que se indica.
Debe tirar el envase, como muy tarde, cuatro semanas después de haberlo abierto por primera vez, aunque todavía queden algunas gotas. Esto ayudará a prevenir las infecciones. Para acordarse, escriba la fecha en que abrió el envase en el espacio previsto en la caja.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de LUMIGAN 0,1 mg/ml
- El principio activo es bimatoprost. Un ml de solución contiene 0,1 mg de bimatoprost.
- Los demás componentes son cloruro de benzalconio (conservante), cloruro sódico, fosfato de sodio dibásico heptahidratado, ácido cítrico monohidratado y agua purificada. Se pueden añadir pequeñas cantidades de ácido clorhídrico o de hidróxido de sodio para mantener normal el nivel de acidez (niveles de pH).
Aspecto de LUMIGAN0,1 mg/mly contenido del envase
LUMIGAN es un colirio en solución incoloro, transparente, en un envase que contiene 1 ó 3 frascos de plástico cada uno de ellos provisto de tapón de rosca. El contenido del frasco sólo llega hasta aproximadamente la mitad y cada frasco contiene 3 ml de solución. El contenido es suficiente para 4 semanas de tratamiento. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación:
AbbVie Deutschland GmbH & Co. KG
Knollstraße
67061 Ludwigshafen
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien Luxembourg/Luxemburg/Nederland Allergan n.v. Tél/Tel: +32 (0)2 351 24 24 | Ísland Actavis Pharmaceuticals Iceland ehf. Sími: +354 550 3300 |
???????? ??????? ???????? ???? ???.: +359 (0) 800 20 280 | Italia Allergan S.p.A Tel: +39 06 509 562 90 |
Ceská republika Allergan CZ s.r.o. Tel: +420 800 188 818 | Latvija Allergan Baltics UAB Tel: +371 676 60 831 |
Danmark/Norge/Suomi/Finland/Sverige Allergan Norden AB Tlf/Puh/Tel: 4580884560 (DK) +47 80 01 04 97 (NO) +358 800 115 003 (FI) +46 (0)8 594 100 00 (SE) | Lietuva Allergan Baltics UAB Tel: +37 052 072 777 |
Deutschland Pharm-Allergan GmbH Tel: +49 69 92038 1050 | Magyarország Allergan Hungary Kft. Tel: +36 80 100 101 |
Eesti Allergan Baltics UAB Tel: + 37 2 634 6109 | Österreich Pharm-Allergan GmbH Tel: +43 1 99460 6355 |
Ελλ?δα/Κ?προς Allergan Hellas Pharmaceuticals S.A. Τηλ: +30 210 74 73 300 | Polska Allergan Sp. z o.o. Tel: +48 22 256 37 00 |
España Allergan S.A.Tel: +34 91 807 6130 | Portugal Profarin Lda. Tel: +351 21 425 3242 |
France Allergan France SAS Tel: +33 (0)1 49 07 83 00 | România Allergan S.R.L. Tel.: +40 21 301 53 02 |
Hrvatska Ewopharma d.o.o. Tel: +385 1 6646 563 | Slovenija Ewopharma d.o.o. Tel: +386 (0) 590 848 40 |
Ireland/MaltaAllergan Pharmaceuticals Ireland Tel: +353 1800 931 787 (IE) +356 27780331 (MT) | Slovenská republika Allergan SK s.r.o. Tel: +421 800 221 223 United Kingdom Allergan Ltd Tel: +44 (0) 1628 494026 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu//
- País de registro
- Precio medio en farmacia7.34 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LUMIGAN 0,1 mg/ml COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 0.3 mg/mlPrincipio activo: BimatoprostFabricante: Tiedra Farmaceutica S.L.Requiere recetaForma farmacéutica: COLIRIO, 0.3 mg/mlPrincipio activo: BimatoprostFabricante: Sifi S.P.A.Requiere recetaForma farmacéutica: COLIRIO, 0,3 mg/mlPrincipio activo: BimatoprostFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para LUMIGAN 0,1 mg/ml COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LUMIGAN 0,1 mg/ml COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















