
LEVETIRACETAM TARBIS FARMA 100 MG/ML SOLUCION ORAL EFG

Cómo usar LEVETIRACETAM TARBIS FARMA 100 MG/ML SOLUCION ORAL EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Levetiracetam Tarbis Farma 100 mg/ml solución oral EFG
levetiracetam
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Levetiracetam Tarbis Farma y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Levetiracetam Tarbis Farma
- Cómo tomar Levetiracetam Tarbis Farma
- Posibles efectos adversos
- Conservación de Levetiracetam Tarbis Farma
- Contenido del envase e información adicional
1. Qué es Levetiracetam Tarbis Farma y para qué se utiliza
Levetiracetam es un medicamento antiepiléptico (un medicamento para el tratamiento de crisis en epilepsia).
Este medicamento se utiliza:
- en solitario en adultos y adolescentes de 16 años de edad o mayores con epilepsia diagnosticada recientemente para tratar una forma de epilepsia. La epilepsia es una enfermedad donde los pacientes tienen ataques (crisis). Levetiracetam se utiliza para la forma de epilepsia en la cual las crisis inicialmente afectan sólo a un lado del cerebro, pero pueden después extenderse a zonas más amplias en los dos lados del cerebro (crisis de inicio parcial con o sin generalización secundaria). Su médico le ha recetado levetiracetam para reducir el número de crisis.
- conjuntamente con otros medicamentos antiepilépticos para tratar:
- las crisis de inicio parcial con o sin generalización en adultos, adolescentes, niños y lactantes a partir de 1 mes de edad.
- las crisis mioclónicas (sacudidas tipo shock, cortas, de un músculo o grupo de músculos) en adultos y adolescentes a partir de 12 años con epilepsia mioclónica juvenil.
- las crisis tónico-clónicas generalizadas primarias (crisis mayores, incluyendo pérdida de
- consciencia) en adultos y adolescentes a partir de 12 años de edad con epilepsia idiopática generalizada (tipo de epilepsia que se piensa que tiene una causa genética).
2. Qué necesita saber antes de empezar a tomar Levetiracetam Tarbis Farma
No tome Levetiracetam Tarbis Farma
- Si es alérgico a levetiracetam, a los derivados de pirrolidona o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico antes de empezar a tomar este medicamento
- Si usted padece problemas de riñón, siga las instrucciones de su médico quien decidirá si debe ajustarle la dosis a tomar.
- Si observa cualquier disminución en el crecimiento de su hijo o un desarrollo de la pubertad inesperado, contacte con su médico.
- Un pequeño número de personas en tratamiento con antiepilépticos tales como levetiracetam han tenido pensamientos de hacerse daño o suicidarse. Si tiene cualquier síntoma de depresión y/o pensamientos suicidas, contacte con su médico.
- Si tiene antecedentes médicos o familiares de ritmo cardíaco irregular (visible en el electrocardiograma), o si tiene una enfermedad y/o toma un tratamiento que le haga(n) propenso a arritmias cardíacas o desequilibrios de sales.
Informe a su médico o farmacéutico si alguno de los siguientes efectos adversos se agrava o dura más de unos pocos días:
- Pensamientos anormales, sensación de irritabilidad o reacciona de forma más agresiva de lo normal o si usted o su familia y amigos notan cambios importantes en el estado de ánimo o comportamiento.
- Agravamiento de la epilepsia
En raras ocasiones, las crisis epilépticas pueden empeorar o producirse con más frecuencia, principalmente durante el primer mes después del inicio del tratamiento o del aumento de la dosis. Si experimenta alguno de estos síntomas nuevos mientras toma este medicamento, acuda a un médico tan pronto como sea posible.
Si experimenta alguno de estos síntomas nuevos mientras toma este medicamento, acuda a un médico tan pronto como sea posible.
Niños y adolescentes
- El tratamiento exclusivo con Levetiracetam Tarbis Farma (monoterapia) no está indicado en niños y adolescentes menores de 16 años.
Otros medicamentos y Levetiracetam Tarbis Farma
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No tome macrogol (medicamento utilizado como laxante) durante una hora antes y una hora después de tomar levetiracetam ya que podría reducir su efecto.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Levetiracetam sólo se puede utilizar durante el embarazo si, después de una cuidadosa evaluación, su médico lo considera necesario.
No debe abandonar su tratamiento sin comentarlo antes con su médico.
No se puede excluir completamente el riesgo de defectos de nacimiento para el bebé.
No se recomienda la lactancia natural durante el tratamiento.
Conducción y uso de máquinas
Levetiracetam puede alterar su capacidad para conducir o manejar herramientas o maquinaria, puesto que puede producirle sensación de sueño. Esto es más probable al inicio del tratamiento o cuando se aumenta la dosis. No debería conducir o utilizar maquinaria hasta que se compruebe que su capacidad para realizar estas actividades no está afectada.
Levetiracetam Tarbis Farma contiene parahidroxibenzoato de metilo (E218), parahidroxibenzoato de propilo (E216) y maltitol (E965).
Parahidroxibenzoato de metilo (E218) y parahidroxibenzoato de propilo (E216) puede causar reacciones alérgicas.
Maltitol: Si su médico le informa que usted tiene intolerancia a algunos azúcares, contacte con su médico antes de tomar este medicamento.
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por ml; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Levetiracetam Tarbis Farma
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Levetiracetam se debe tomar dos veces al día, una vez por la mañana y otra por la noche, aproximadamente a la misma hora cada día.
Tome la solución oral según las instrucciones de su médico.
Monoterapia (desde 16 años de edad)
Adultos (≥18 años) y adolescentes (desde 16 años de edad):
Para pacientes a partir de 4 años de edad, medir la dosis adecuada utilizando la jeringa de 10 ml incluida en la caja.
Dosis recomendada: levetiracetam se toma dos veces al día, en dos dosis iguales, cada dosis individual entre 5 ml (500 mg) y 15 ml (1500 mg).
Cuando empiece a tomar Levetiracetam, su médico le prescribirá una dosis inferior durante dos semanas antes de administrarle la dosis diaria más baja.
Terapia concomitante
Dosis en adultos y adolescentes (de 12 a 17 años):
Para pacientes a partir de 4 años de edad, medir la dosis adecuada utilizando la jeringa de 10 ml incluida en la caja.
Dosis recomendada: Levetiracetam se toma dos veces al día, en dos dosis iguales, cada dosis individual entre 5 ml (500 mg) y 15 ml (1500 mg).
Dosis en niños a partir de 6 meses de edad:
Su médico le prescribirá la forma farmacéutica de levetiracetam más apropiada según la edad, el peso y la dosis.
Para niños de 6 meses a 4 años de edad, medir la dosis adecuada utilizando la jeringa de 3 ml incluida en la caja.
Para niños mayores de 4 años de edad, medir la dosis adecuada utilizando la jeringa de 10 ml incluida en la caja.
Dosis recomendada: Levetiracetam se toma dos veces al día, en dos dosis iguales, cada dosis individual entre 0,1 ml (10 mg) y 0,3 ml (30 mg) por kg de peso corporal del niño (ver en la siguiente tabla los ejemplos de dosis).
Dosis en niños a partir de 6 meses de edad:
Peso | Dosis inicial: 0,1 ml/kg dos veces al día | Dosis máxima: 0,3 ml/kg dos veces al día |
6 kg | 0,6 ml dos veces al día | 1,8 ml dos veces al día |
8 kg | 0,8 ml dos veces al día | 2,4 ml dos veces al día |
10 kg | 1 ml dos veces al día | 3 ml dos veces al día |
15 kg | 1,5 ml dos veces al día | 4,5 ml dos veces al día |
20 kg | 2 ml dos veces al día | 6 ml dos veces al día |
25 kg | 2,5 ml dos veces al día | 7,5 ml dos veces al día |
A partir de 50 kg | 5 ml dos veces al día | 15 ml dos veces al día |
Dosificación en lactantes (de 1 mes a menos de 6 meses):
Para lactantes de 1 mes a menos de 6 meses de edad, medir la dosis adecuada utilizando la jeringa de 1 ml incluida en la caja.
Dosis recomendada: Levetiracetam se toma dos veces al día, en dos dosis iguales, cada dosis individual entre 0,07 ml (7 mg) y 0,21 ml (21 mg) por kg de peso corporal del lactante (ver en la siguiente tabla los ejemplos de dosis).
Dosis en lactantes (de 1 mes a menos de 6 meses de edad):
Peso | Dosis inicial: 0,07 ml/kg dos veces al día | Dosis máxima: 0,21 ml/kg dos veces al día |
4 kg | 0,3 ml dos veces al día | 0,85 ml dos veces al día |
5 kg | 0,35 ml dos veces al día | 1,05 ml dos veces al día |
6 kg | 0,45 ml dos veces al día | 1,25 ml dos veces al día |
7 kg | 0,5 ml dos veces al día | 1,5 ml dos veces al día |
Forma de administración:
Este medicamento es para uso oral. Después de medir la dosis correcta con la jeringa adecuada, Levetiracetam solución oral se puede diluir en un vaso de agua o en un biberón. Puede tomar Levetiracetam con o sin alimentos. Tras la administración oral de levetiracetam se puede apreciar su sabor amargo.
Instrucciones sobre cómo usar la jeringa:
- Abrir el frasco: apretar el tapón y desenroscar en sentido contrario a las agujas del reloj (figura 1)
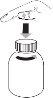
- Siga estos pasos la primera vez que tome Levetiracetam:
- Retire el adaptador de la jeringa oral (figura 2).
- Inserte el adaptador en la parte superior del frasco (figura 3). Asegúrese de que esté bien colocado. No es necesario retirar el adaptador después de su uso.
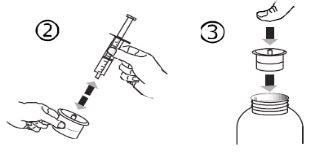
- Siga estos pasos cada vez que tome Levetiracetam
- Introduzca la jeringa oral en la abertura del adaptador (figura 4).
- Voltee el frasco boca abajo (figura 5).
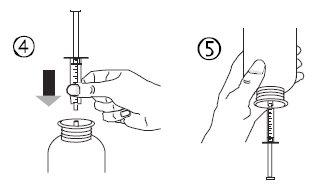
- Sostenga el frasco boca abajo con una mano y con la otra mano llene la jeringa oral.
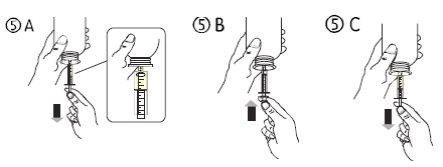
- Tire del émbolo hacia abajo para llenar la jeringa oral con una pequeña cantidad de solución (figura 5A).
- Luego, empuje el émbolo hacia arriba para eliminar posibles burbujas de aire (figura 5B).
- Tire del émbolo hacia abajo hasta la marca de dosis en mililitros (ml) indicada en la jeringa oral y prescrita por su médico (figura 5C). El émbolo puede retroceder en el cilindro en la primera dosis, por lo que debe asegurarse de mantener el émbolo en su posición hasta desconectar la jeringa del frasco.
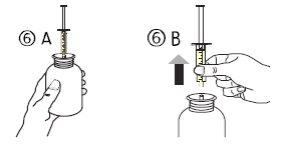
- Poner el frasco boca arriba (figura 6A). Retirar la jeringa del adaptador (figura 6B).
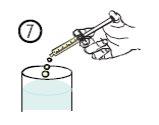
- Vaciar el contenido de la jeringa en un vaso de agua o en un biberón, bajando el émbolo hasta el final de la jeringa (figura 7).
- Beber el contenido del vaso o del biberón entero.
- Cerrar el frasco con el tapón de rosca de plástico (no es necesario retirar el adaptador).
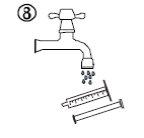
- Para limpiar la jeringa, enjuáguela solo con agua fría, moviendo el émbolo varias veces hacia arriba y hacia abajo para absorber y expulsar el agua, sin separar los dos componentes (figura 8).
- Guarde el frasco, la jeringa oral y el prospecto en la caja.
Duración del tratamiento:
- Levetiracetam se utiliza como un tratamiento crónico. Debe continuar con el tratamiento con levetiracetam durante el tiempo indicado por su médico.
- No deje su tratamiento sin la recomendación de su médico ya que pueden aumentar sus crisis.
Si toma más Levetiracetam Tarbis Farma del que debe
Los posibles efectos adversos de una sobredosis de levetiracetam son somnolencia, agitación, agresividad, disminución de la alerta, inhibición de la respiración y coma.
Contacte con su médico si ha tomado más solución oral de la que debiera. Su médico establecerá el mejor tratamiento posible de la sobredosis.
En caso de sobredosis o ingestión accidental, consulte con su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 915 620 420, indicando el medicamento y la cantidad utilizada.
Si olvidó tomar Levetiracetam Tarbis Farma:
Contacte con su médico si ha dejado de tomar una o más dosis.
No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Levetiracetam Tarbis Farma:
La finalización del tratamiento con levetiracetam debe efectuarse de forma gradual para evitar un incremento de las crisis. Si su médico decide parar su tratamiento con levetiracetam, él/ella le dará las instrucciones para la retirada gradual de levetiracetam.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, levetiracetam puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico inmediatamente, o vaya al servicio de urgencias de su hospital más cercano si experimenta:
- debilidad, mareo o dificultad para respirar, ya que éstos pueden ser signos de una reacción alérgica (anafiláctica) grave
- hinchazón de la cara, labios, lengua o garganta (edema de Quincke)
- síntomas de gripe y erupción en la cara seguido de una erupción prolongada con temperatura elevada, niveles de enzimas hepáticos elevados en tests sanguíneos y un aumento en un tipo de células blancas sanguíneas (eosinofilia) y ganglios linfáticos agrandados y la afectación de otros órganos del cuerpo (Reacción de hipersensibilidad al medicamento con eosinofilia y síntomas sistémicos (DRESS))
- síntomas como bajo volumen de orina, cansancio, nauseas, vómitos, confusión e hinchazón de piernas, brazos o pies, ya que puede ser un signo de disminución súbita de la función renal
- una erupción cutánea que puede formar ampollas y puede aparecer como pequeñas dianas (puntos centrales oscuros rodeados por un área más pálida, con un anillo oscuro alrededor del borde) (eritema multiforme)
- una erupción generalizada con ampollas y descamación de la piel, especialmente alrededor de la boca, nariz, ojos y genitales (síndrome de Stevens-Johnson)
- una forma más grave que causa descamación de la piel en más del 30% de la superficie corporal (necrólisis epidérmica tóxica)
- signos de cambios mentales graves o si alguien a su alrededor nota signos de confusión, somnolencia (adormecimiento), amnesia (pérdida de memoria), deterioro de la memoria (olvidos), comportamiento anormal u otros signos neurológicos incluyendo movimientos involuntarios o incontrolados. Éstos pueden ser síntomas de encefalopatía.
Los efectos adversos notificados más frecuentemente son nasofaringitis, somnolencia (sensación de sueño), dolor de cabeza, fatiga y mareo. Los efectos adversos como sensación de sueño, sensación de debilidad y mareos pueden ser más frecuentes cuando se inicia el tratamiento o se aumenta la dosis. Sin embargo, estos efectos adversos deben disminuir con el tiempo.
Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- nasofaringitis;
- somnolencia (sensación de sueño), dolor de cabeza.
Frecuentes:pueden afectar hasta 1 de cada 10 personas
- anorexia (pérdida de apetito);
- depresión, hostilidad o agresividad, ansiedad, insomnio, nerviosismo o irritabilidad;
- convulsiones, trastorno del equilibrio, mareos (sensación de inestabilidad), letargo (falta de energía y entusiasmo), temblor (temblor involuntario);
- vértigo (sensación de rotación);
- tos;
- dolor abdominal, diarrea, dispepsia (digestión pesada, ardor y acidez),vómitos, náuseas;
- erupción en la piel;
- astenia/fatiga (sensación de debilidad).
Poco frecuentes:pueden afectar hasta 1 de cada 100 personas
- disminución del número de plaquetas, disminución de los glóbulos blancos;
- pérdida de peso, aumento de peso;
- intento de suicidio y pensamientos suicidas, alteraciones mentales, comportamiento anormal, alucinaciones, cólera, confusión, ataque de pánico, inestabilidad emocional/cambios de humor, agitación;
- amnesia (pérdida de memoria), deterioro de la memoria (falta de memoria), coordinación anormal/ataxia (coordinación de los movimientos alterada), parestesia (hormigueo), alteraciones de la atención (pérdida de concentración);
- diplopía (visión doble), visión borrosa;
- valores elevados/anormales en las pruebas sobre la funcionalidad del hígado;
- pérdida de cabello, eczema, picor;
- debilidad muscular, mialgia (dolor muscular);
- lesión.
Raros:pueden afectar hasta 1 de cada 1.000 personas
- infección;
- disminución de todos los tipos de células sanguíneas;
- reacciones alérgicas graves (DRESS, reacción anafiláctica (reacción alérgica importante y grave), edema de Quincke (hinchazón de cara, labios, lengua y garganta));
- disminución de la concentración de sodio en sangre;
- suicidio, trastornos de la personalidad (problemas de comportamiento), pensamiento anormal (pensamiento lento, dificultad para concentrarse);
- delirio;
- encefalopatía (ver subsección “Informe a su médico inmediatamente” para ver una descripción detallada de los síntomas);
- las crisis epilépticas pueden empeorar o producirse con más frecuencia;
- espasmos musculares incontrolables que afectan a la cabeza, al torso y a las extremidades, dificultad para controlar los movimientos, hipercinesia (hiperactividad);
- cambio del ritmo cardiaco (electrocardiograma);
- pancreatitis (inflamación del páncreas);
- insuficiencia hepática, hepatitis (inflamación del hígado);
- disminución súbita de la función renal;
- erupción cutánea, que puede dar lugar a ampollas que pueden aparecer como pequeñas dianas (puntos centrales oscuros rodeados por un área más pálida, con un anillo oscuro alrededor del borde) (eritema multiforme), una erupción generalizada con ampollas y descamación de la piel, especialmente alrededor de la boca, nariz, ojos y genitales (síndrome de Stevens-Johnson) y una forma más grave que causa descamación de la piel en más del 30% de la superficie corporal (necrólisis epidérmica tóxica);
- rabdomiólisis (rotura del tejido muscular) y aumento de creatinfosfoquinasa sanguínea asociado. La prevalencia es significativamente mayor en pacientes japoneses en comparación con pacientes no japoneses;
- cojera o dificultad para caminar.
- combinación de fiebre, rigidez muscular, presión arterial y frecuencia cardíaca inestables, confusión, estado de bajo nivel de conciencia (pueden ser signos de un trastorno llamado síndrome neuroléptico maligno). La prevalencia es significativamente mayor en pacientes japoneses en comparación con pacientes no japoneses.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También pueden comunicarse directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano website: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Levetiracetam Tarbis Farma
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el cartón después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que yano necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Levetiracetam Tarbis Farma
El principio activo es levetiracetam.
Cada ml contiene 100 mg de levetiracetam.
Los demás componentes son: maltitol líquido (E965), Glicerol (E422), parahidroxibenzoato de metilo (E218), parahidroxibenzoato de propilo (E216), citrato de sodio monohidrato, ácido cítrico (dihidrato), glicirrizato de amonio, acesulfamo potásico (E950), aroma de uva, agua purificada
Aspecto del producto y contenido del envase
Levetiracetam Tarbis Farma 100 mg/ml solución oral EFG es un líquido transparente e incoloro.
Tamaños de envase:
Frasco de vidrio ámbar de 300 ml (tipo III) (conteniendo 300 ml de solución oral) con tapón blanco a prueba de niños (polietileno), acompañado de una jeringa oral graduada de 10 ml (polipropileno) y un adaptador para la jeringa (polietileno), todo ello contenido en una caja de cartón.
Frasco de vidrio ámbar de 200 ml (tipo III) (conteniendo 150 ml de solución oral) con tapón blanco a prueba de niños (polietileno), acompañado de una jeringa oral graduada de 3 ml (polipropileno) y un adaptador para la jeringa (polietileno), todo ello contenido en una caja de cartón.
Frasco de vidrio ámbar de 200 ml (tipo III) (conteniendo 150 ml de solución oral) con tapón blanco a prueba de niños (polietileno), acompañado de una jeringa oral graduada de 1 ml (polipropileno) y un adaptador para la jeringa (polietileno), todo ello contenido en una caja de cartón.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Tarbis Farma S.L.
Gran Vía Carlos III, 94
08028 Barcelona
España
Responsable de la fabricación
Amarox Pharma B.V.
Rouboslaan 32
Voorschoten, 2252TR
Países Bajos
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con siguientes nombres:
Países Bajos: Levetiracetam Amarox 100 mg/ml drank
Alemania: Levetiracetam Amarox 100 mg/ml Lösung zum Einnehmen
España: Levetiracetam Tarbis Farma 100 mg/ml solución oral EFG
Fecha de la última revisión de este prospecto:Marzo 2024
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia28.32 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LEVETIRACETAM TARBIS FARMA 100 MG/ML SOLUCION ORAL EFGForma farmacéutica: INYECTABLE PERFUSION, 100 mgPrincipio activo: LevetiracetamFabricante: Ucb PharmaRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 100 mg/mlPrincipio activo: LevetiracetamFabricante: Ucb PharmaRequiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 100 mgPrincipio activo: LevetiracetamFabricante: Ucb PharmaRequiere receta
Médicos online para LEVETIRACETAM TARBIS FARMA 100 MG/ML SOLUCION ORAL EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LEVETIRACETAM TARBIS FARMA 100 MG/ML SOLUCION ORAL EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes








