
LENALIDOMIDA TEVA 20 MG CAPSULAS DURAS EFG

Cómo usar LENALIDOMIDA TEVA 20 MG CAPSULAS DURAS EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto:información para el paciente
Lenalidomida Teva 5 mg cápsulas duras EFG
Lenalidomida Teva 10 mg cápsulas duras EFG
Lenalidomida Teva 15 mg cápsulas duras EFG
Lenalidomida Teva 20 mg cápsulas duras EFG
Lenalidomida Teva 25 mg cápsulas duras EFG
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Lenalidomida Teva y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Lenalidomida Teva
- Cómo tomar Lenalidomida Teva
- Posibles efectos adversos
- Conservación de Lenalidomida Teva
- Contenido del envase e información adicional
1. Qué es Lenalidomida Teva y para qué se utiliza
Qué esLenalidomida Teva
Lenalidomida Teva contiene el principio activo “lenalidomida”. Este medicamento pertenece a un grupo de
medicamentos que afectan a cómo funciona el sistema inmunitario.
Para qué se utiliza Lenalidomida Teva
Lenalidomida Teva se usa en adultos para:
- Mieloma múltiple
- Síndromes mielodisplásicos (SMD)
- Linfoma de células del manto (LCM)
- Linfoma folicular
Mieloma múltiple
El mieloma múltiple es un tipo de cáncer que afecta a un tipo concreto de glóbulos blancos de la sangre, que se llaman células plasmáticas. Estas células se acumulan en la médula ósea y se multiplican, pasando a estar fuera de control. Esto puede dañar los huesos y los riñones.
El mieloma múltiple por lo general no tiene cura. Sin embargo, se pueden reducir mucho los signos y
síntomas o pueden desaparecer durante un periodo de tiempo. A esto se le llama “remisión”.
Mieloma múltiple de nuevo diagnóstico: en pacientes que se han sometido a un trasplante de médula ósea
Lenalidomida Teva se utiliza como tratamiento de mantenimiento después de recuperarse de manera adecuada tras un trasplante de médula ósea.
Mieloma múltiple de nuevo diagnóstico: en pacientes que no se puedan tratar con un trasplante de médula ósea
Lenalidomida Teva se toma con otros medicamentos, entre ellos:
- un medicamento de quimioterapia llamado “bortezomib”
- un antiinflamatorio llamado “dexametasona”
- un medicamento de quimioterapia llamado “melfalán” y
- un inmunosupresor llamado “prednisona”.
Tomará estos medicamentos al comenzar el tratamiento y luego continuará tomando Lenalidomida Teva solo.
Si tiene 75 años o más o tiene problemas de riñón de moderados a graves, su médico le controlará
cuidadosamente antes de iniciar el tratamiento.
Mieloma múltiple: en pacientes tratados anteriormente
Lenalidomida Teva se toma junto con un antiinflamatorio llamado “dexametasona”.
Lenalidomida Teva puede frenar el empeoramiento de los signos y síntomas del mieloma múltiple. También ha demostrado retrasar la reaparición del mieloma múltiple tras el tratamiento.
Síndromes mielodisplásicos (SMD)
Los SMD son un grupo de muchas enfermedades diferentes de la sangre y de la médula ósea. Las células de la sangre se vuelven anormales y no funcionan correctamente. Los pacientes pueden experimentar una variedad de signos y síntomas entre los que están un recuento bajo de glóbulos rojos (anemia), la necesidad de transfusión de sangre y el riesgo de infección.
Lenalidomida Teva se utiliza para tratar a pacientes adultos diagnosticados de SMD, cuando todos los siguientes puntos sean aplicables:
- necesita transfusiones de sangre periódicamente para tratar los niveles bajos de glóbulos rojos (“anemia dependiente de transfusiones”);
- tiene una anomalía de las células de la médula ósea llamada “anomalía citogenética de deleción 5q aislada”. Esto significa que su cuerpo no produce un número suficiente de células sanguíneas sanas;
- otros tratamientos que ha utilizado anteriormente no son adecuados o no funcionan lo suficientemente bien.
Lenalidomida Teva puede aumentar el número de glóbulos rojos sanos que produce el organismo al reducir el número de células anormales:
- esto puede reducir el número de transfusiones de sangre necesarias. Es posible que no se necesiten transfusiones.
Linfoma de células del manto (LCM)
El LCM es un cáncer de una parte del sistema inmunológico (el tejido linfático). Afecta a un tipo de glóbulos blancos de la sangre llamados “linfocitos B” o células B. El LCM es una enfermedad en la que las células B crecen sin control y se acumulan en el tejido linfático, la médula ósea o la sangre.
Lenalidomida Teva se utiliza en monoterapia para tratar a pacientes adultos que han recibido tratamiento anteriormente con otros medicamentos.
Linfoma folicular (LF)
El LF es un cáncer de crecimiento lento que afecta a los linfocitos B. Estos son un tipo de glóbulos blancos que ayudan al organismo a combatir las infecciones. Cuando una persona sufre LF puede almacenar demasiados de estos linfocitos B en la sangre, la médula ósea, los nódulos linfáticos y el bazo.
Lenalidomida Teva se utiliza con otro medicamento llamado “rituximab” para el tratamiento de pacientes adultos que han recibido tratamiento previo para el linfoma folicular.
Cómo actúa Lenalidomida Teva
Lenalidomida Teva actúa afectando al sistema inmunitario del organismo y atacando directamente al cáncer.
Actúa de diversas formas:
- detiene el desarrollo de las células cancerosas
- detiene el crecimiento de los vasos sanguíneos en el cáncer
- estimula parte del sistema inmunitario para que ataque a las células cancerosas.
2. Qué necesita saber antes de empezar a tomar Lenalidomida Teva
Debe leer el prospecto de todos los medicamentos que vaya a tomar en combinación con Lenalidomida Teva antes de empezar el tratamiento con Lenalidomida Teva.
No tome Lenalidomida Teva
- si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, ya que se espera que Lenalidomida Teva sea perjudicial para el feto(ver sección 2, “Embarazo, lactancia y anticoncepción: información para mujeres y hombres”).
- si puede quedarse embarazada, a menos que siga todas las medidas necesarias para evitarlo (ver sección 2, “Embarazo, lactancia y anticoncepción: información para mujeres y hombres”). Si puede quedarse embarazada, su médico anotará con cada receta que se han tomado todas las medidas necesarias y le proporcionará esta confirmación.
- si es alérgico a lenalidomida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Si cree que puede ser alérgico, consulte a su médico.
Si alguna de estas condiciones es aplicable a usted, no tome Lenalidomida Teva. En caso de duda, consulte a su médico.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a tomar Lenalidomida Teva si
- ha tenido alguna vez coágulos de sangre; durante el tratamiento, tiene un mayor riesgo de presentar coágulos de sangre en las venas y en las arterias.
- tiene algún signo de infección, como tos o fiebre.
- tiene o ha tenido previamente una infección viral, especialmente infección por hepatitis B, varicela zoster o VIH. En caso de duda, consulte a su médico. El tratamiento con Lenalidomida Teva puede hacer que el virus se vuelva activo de nuevo en los pacientes portadores del virus. Esto da lugar a la reaparición de la infección. Su médico debe comprobar si ha tenido alguna vez una infección por hepatitis B.
- tiene problemas de riñón; su médico puede ajustarle la dosis de Lenalidomida Teva.
- ha tenido un ataque al corazón, alguna vez ha tenido un coágulo de sangre, o si fuma, tiene la tensión arterial alta o los niveles de colesterol altos.
- ha tenido una reacción alérgica mientras utilizaba talidomida (otro medicamento que se utiliza para tratar el mieloma múltiple), como por ejemplo erupción cutánea, picor, hinchazón, mareos o problemas respiratorios.
- ha experimentado en el pasado una combinación de cualquiera de los síntomas siguientes: erupción generalizada, enrojecimiento de la piel, temperatura corporal alta, síntomas de tipo gripal, aumento de las enzimas hepáticas, anomalías en la sangre (eosinofilia), nódulos linfáticos engrosados (son signos de una reacción cutánea grave llamada reacción al fármaco con eosinofilia y síntomas sistémicos, que también se conoce como DRESS por sus siglas en inglés o síndrome de hipersensibilidad al fármaco) (ver también la sección 4 “Posibles efectos adversos”).
Si alguna de las alteraciones anteriores es aplicable a usted, informe a su médico, farmacéutico o enfermero antes de empezar el tratamiento.
En cualquier momento durante o después del tratamiento, informe a su médico o enfermero inmediatamente si:
- presenta visión borrosa, pérdida de la visión o visión doble, dificultad para hablar, debilidad en un brazo o una pierna, un cambio en la forma de caminar o problemas de equilibrio, entumecimiento persistente, disminución de la sensibilidad o pérdida de sensibilidad, pérdida de memoria o confusión. Todos ellos pueden ser síntomas de una enfermedad cerebral grave y potencialmente mortal conocida como leucoencefalopatía multifocal progresiva (LMP). Si tiene alguno de estos síntomas antes de empezar el tratamiento con Lenalidomida Teva, informe a su médico si observa algún cambio en estos síntomas.
- presenta falta de aire, cansancio, mareo, dolor en el pecho, latido cardíaco más rápido o hinchazón en las piernas o los tobillos. Estos pueden ser síntomas de una afección grave conocida como hipertensión pulmonar (ver sección 4).
Análisis y pruebas
Antes de iniciar el tratamiento con Lenalidomida Teva y durante el mismo, le harán análisis de sangre con regularidad. Esto se debe a que Lenalidomida Teva puede causar una disminución de las células de la sangre que ayudan a luchar contra las infecciones (glóbulos blancos) y de las que participan en la coagulación (plaquetas).
Su médico le solicitará que le hagan un análisis de sangre:
- antes del tratamiento
- cada semana, durante las 8 primeras semanas de tratamiento
- posteriormente, por lo menos cada mes.
Puede que se le evalúe para detectar signos de problemas cardiopulmonares antes y durante el tratamiento con lenalidomida.
Para pacientes con SMD que tomen Lenalidomida Teva
Si tiene un SMD, puede ser más propenso a padecer una enfermedad más avanzada llamada leucemia mieloide aguda (LMA). Además, se desconoce cómo afecta Lenalidomida Teva a las posibilidades de que desarrolle LMA. Su médico, por tanto, le podrá hacer analíticas para detectar signos que puedan predecir mejor la posibilidad de que desarrolle LMA durante el tratamiento con Lenalidomida Teva.
Para pacientes con LCM que tomen Lenalidomida Teva
Su médico le solicitará que le hagan un análisis de sangre:
- antes del tratamiento
- cada semana durante las primeras 8 semanas (2 ciclos) de tratamiento
- a continuación, cada 2 semanas en los ciclos 3 y 4 (ver sección 3 “Ciclo de tratamiento” para obtener más información)
- después de esto se hará al comienzo de cada ciclo y
- al menos cada mes.
Para pacientes con LF que tomen Lenalidomida Teva
Su médico le solicitará que le hagan un análisis de sangre:
- antes del tratamiento
- cada semana durante las primeras 3 semanas (1 ciclo) de tratamiento
- a continuación, cada 2 semanas en los ciclos 2 a 4 (ver sección 3 “Ciclo de tratamiento” para obtener más información)
- después de esto se hará al comienzo de cada ciclo y
- al menos cada mes.
Su médico puede comprobar si tiene una cantidad total del tumor alta en el cuerpo, incluida la médula ósea. Esto podría dar lugar a una enfermedad en la que los tumores se descomponen y producen niveles inusuales de sustancias químicas en la sangre que, a su vez, pueden originar insuficiencia renal (esta enfermedad se llama “síndrome de lisis tumoral”).
Su médico puede examinarle para comprobar si se han producido cambios en su piel, tales como manchas rojas o erupciones cutáneas.
Su médico puede ajustar la dosis de Lenalidomida Teva o interrumpir su tratamiento, dependiendo de los resultados de sus análisis de sangre y de su estado general. Si es un paciente de nuevo diagnóstico, su médico puede evaluar también su tratamiento en función de su edad y de otras afecciones que ya tenga.
Donación de sangre
No debe donar sangre durante el tratamiento ni durante al menos 7 días después del final del tratamiento.
Niños y adolescentes
No está recomendado el uso de Lenalidomida Teva en niños y adolescentes menores de 18 años.
Personas de edad avanzada y personas con problemas renales
Si tiene 75 años o más o tiene problemas renales de moderados a graves, su médico le examinará detenidamente antes de iniciar el tratamiento.
Otros medicamentos y Lenalidomida Teva
Informe a su médico o enfermero si está tomando o ha tomado recientemente cualquier otro medicamento. Esto se debe a que Lenalidomida Teva puede afectar a la forma en que funcionan otros medicamentos. Además, algunos medicamentos pueden afectar a la forma en que funciona Lenalidomida Teva.
En concreto, informe a su médico o enfermero si está tomando alguno de los siguientes medicamentos:
- algunos medicamentos que se utilizan para prevenir el embarazo, tales como los anticonceptivos orales, ya que pueden dejar de funcionar
- algunos medicamentos que se utilizan para problemas de corazón, tales como la digoxina
- algunos medicamentos que se utilizan para adelgazar la sangre, tales como la warfarina
Embarazo, lactancia y anticoncepción: información para mujeres y hombres
Embarazo
Mujeres que toman Lenalidomida Teva
- No debe tomar Lenalidomida Teva si está embarazada, ya que se espera que sea perjudicial para el feto.
- No se debe quedar embarazada mientras toma Lenalidomida Teva. Por lo tanto, tiene que usar métodos anticonceptivos eficaces si existe la posibilidad de que pueda quedarse embarazada (ver “Anticoncepción”).
- Si se queda embarazada durante el tratamiento con Lenalidomida Teva, debe interrumpir el tratamiento e informar inmediatamente a su médico.
Hombres que toman Lenalidomida Teva
- Si su pareja se queda embarazada mientras usted toma Lenalidomida Teva, debe informar inmediatamente a su médico. Es recomendable que su pareja solicite consejo médico.
- Usted también debe utilizar métodos anticonceptivos efectivos (ver “Anticoncepción”).
Lactancia
No debe dar el pecho mientras tome Lenalidomida Teva, ya que se desconoce si Lenalidomida Teva pasa a la leche materna.
Anticoncepción
Para las mujeres que toman Lenalidomida Teva
Antes de comenzar el tratamiento, pregunte a su médico si tiene la capacidad de quedarse embarazada, aunque crea que esto es poco probable.
Si puede quedarse embarazada
- le harán pruebas de embarazo bajo supervisión médica (antes de cada tratamiento, al menos cada 4 semanas durante el tratamiento y al menos 4 semanas después de finalizar el tratamiento) excepto que se haya confirmado el cierre de las trompas de Falopio para que los óvulos no lleguen al útero (ligadura de trompas)
Y
- debe usar métodos anticonceptivos efectivos desde al menos 4 semanas antes de iniciar el tratamiento, durante el tratamiento y hasta al menos 4 semanas después de finalizar el tratamiento. Su médico le aconsejará sobre los métodos anticonceptivos más adecuados.
Para los hombres que toman Lenalidomida Teva
Lenalidomida pasa al semen humano. Si su pareja está embarazada o puede quedarse embarazada y no utiliza ningún método anticonceptivo eficaz, usted debe utilizar preservativos durante el tratamiento y hasta al menos 7 días después de finalizar el tratamiento, incluso si se ha sometido a una vasectomía. No debe donar semen o esperma durante el tratamiento ni durante, al menos, 7 días después del final del tratamiento.
Conducción y uso de máquinas
No conduzca ni utilice máquinas si se siente mareado, cansado, adormilado, tiene vértigo o visión borrosa después de tomar Lenalidomida Teva.
Lenalidomida Teva contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por cápsula; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Lenalidomida Teva
Lenalidomida Teva se lo debe administrar un profesional sanitario con experiencia en el tratamiento del mieloma múltiple, SMD, LCM o LF.
- Cuando Lenalidomida Teva se utiliza para el tratamiento del mieloma múltiple en pacientes que no se pueden tratar con un trasplante de médula ósea o se han sometido a otros tratamientos antes, se toma con otros medicamentos (ver sección 1 “Para qué se utiliza Lenalidomida Teva”).
- Cuando Lenalidomida Teva se utiliza para el tratamiento del mieloma múltiple en pacientes que se han sometido a un trasplante de médula ósea o para tratar pacientes con SMD o LCM, se toma solo.
- Cuando Lenalidomida Teva se utiliza para el tratamiento del linfoma folicular, se toma con otro medicamento llamado “rituximab”.
Siga exactamente las instrucciones de administración de Lenalidomida Teva indicadas por su médico. En caso de duda, consulte a su médico o farmacéutico.
Si está tomando Lenalidomida Teva junto con otros medicamentos, debe consultar el prospecto de esos otros medicamentos para obtener información adicional sobre su uso y sus efectos.
Ciclo de tratamiento
Lenalidomida Teva se toma ciertos días durante el periodo de 3 semanas (21 días).
- Un “ciclo de tratamiento” consta de 21 días.
- Dependiendo del día del ciclo, tomará uno o más medicamentos. Sin embargo, algunos días no tomará ningún medicamento.
- Después de terminar cada ciclo de 21 días, debe comenzar un nuevo “ciclo” durante los siguientes 21 días.
O
Lenalidomida Teva se toma ciertos días durante el periodo de 4 semanas (28 días).
- Un “ciclo de tratamiento” consta de 28 días.
- Dependiendo del día del ciclo, tomará uno o más medicamentos. Sin embargo, algunos días no tomará ningún medicamento.
- Después de terminar cada ciclo de 28 días, debe comenzar un nuevo “ciclo” durante los siguientes 28 días.
Cuánto Lenalidomida Teva tomar
Antes de comenzar el tratamiento, su médico le indicará:
- qué cantidad de Lenalidomida Teva debe tomar
- qué cantidad de los otros medicamentos debe tomar junto con Lenalidomida Teva, en su caso
- qué días del ciclo de tratamiento debe tomar cada medicamento.
Cómo y cuándo tomar Lenalidomida Teva
- trague las cápsulas enteras, preferiblemente con agua.
- no rompa, abra ni mastique las cápsulas. En el caso de que el polvo de una cápsula rota de Lenalidomida Teva entre en contacto con la piel, lave la piel de forma inmediata y cuidadosa con agua y jabón.
- los profesionales sanitarios, cuidadores y familiares se deben poner guantes desechables cuando manipulen el blíster o la cápsula. Posteriormente, se deben quitar los guantes con cuidado para evitar la exposición cutánea, introducirlos en una bolsa de plástico de polietileno sellable y eliminarlos de acuerdo con los requisitos locales. A continuación, se deben lavar bien las manos con agua y jabón. Las mujeres embarazadas o que sospechen que puedan estarlo no deben manipular el blíster ni la cápsula.
- las cápsulas pueden tomarse con o sin alimentos.
- debe tomar Lenalidomida Teva aproximadamente a la misma hora en los días programados.
Toma de este medicamento
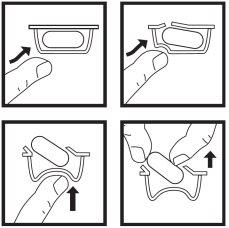
Para sacar la cápsula del blister:
- presione solo un extremo de la cápsula para que salga a través de la lámina.
- no presione en el centro de la cápsula ya que podría romperla.
Duración del tratamiento con Lenalidomida Teva
Lenalidomida Teva se toma en ciclos de tratamiento, cada ciclo dura 21 o 28 días (ver “Ciclo de tratamiento” más arriba). Debe continuar los ciclos de tratamiento hasta que su médico le comunique que interrumpa el tratamiento.
Si toma más Lenalidomida Teva del que debe
Si toma más Lenalidomida Teva del que le han recetado, informe inmediatamente a su médico.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico, a su farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Lenalidomida Teva
Si olvida tomar Lenalidomida Teva a su hora habitual y
- han transcurrido menos de 12 horas - tome la cápsula inmediatamente.
- han transcurrido más de 12 horas - no tome la cápsula. Tome la próxima cápsula al día siguiente a la hora habitual.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Lenalidomida Teva puede producir efectos adversos, aunque no todas las personas los sufran.
Si experimenta alguno de los siguientes efectos adversos graves, interrumpa el tratamiento con Lenalidomida Teva y acuda a un médico inmediatamente, porque podría requerir tratamiento médico de urgencia:
- Urticaria, erupciones, hinchazón de los ojos, boca o cara, dificultad para respirar o picor, que pueden ser síntomas de tipos graves de reacciones alérgicas llamadas angioedema y reacción anafiláctica
- Reacción alérgica grave que puede comenzar como una erupción en una zona, pero se extiende produciendo una pérdida importante de piel por todo el cuerpo (síndrome de Stevens-Johnson y/o necrólisis epidérmica tóxica).
- Erupción generalizada, temperatura corporal alta, aumento de las enzimas hepáticas, anomalías en la sangre (eosinofilia), nódulos linfáticos engrosados y efectos sobre otros órganos del cuerpo (reacción al fármaco con eosinofilia y síntomas sistémicos, que también se conoce como síndromeDRESS o síndrome de hipersensibilidad al fármaco). Ver también sección 2.
Consulte inmediatamente a su médico si nota cualquiera de los siguientes efectos adversos graves:
- Fiebre, escalofríos, dolor de garganta, tos, úlceras bucales o cualquier otro síntoma de infección incluyendo en el torrente sanguíneo (sepsis)
- Hemorragia o hematoma no debidos a una lesión
- Dolor en el pecho o en las piernas
- Dificultad respiratoria
- Dolor óseo, dolor muscular, confusión o cansancio que pueden deberse a niveles altos de calcio en la sangre.
Lenalidomida Teva puede reducir el número de glóbulos blancos que combaten las infecciones y también de las células de la sangre que ayudan a coagular la sangre (plaquetas), lo que puede provocar trastornos hemorrágicos como sangrados de nariz y moratones. Lenalidomida Teva también puede causar coágulos de sangre en las venas (trombosis).
Otros efectos adversos
Es importante señalar que un número reducido de pacientes puede desarrollar otros tipos de cáncer, y es posible que este riesgo aumente con el tratamiento con Lenalidomida Teva, por lo tanto, su médico debe evaluar cuidadosamente los beneficios y los riesgos al recetarle Lenalidomida Teva.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- Una disminución del número de glóbulos rojos lo que puede producir anemia que da lugar a cansancio y debilidad
- Erupción cutánea, picor
- Calambres musculares, debilidad muscular, dolor muscular, molestias musculares, dolor óseo, dolor de las articulaciones, dolor de espalda, dolor en las extremidades.
- Hinchazón generalizada que incluye hinchazón de los brazos y las piernas
- Debilidad, cansancio
- Fiebre y síntomas seudogripales que incluyen fiebre, dolor muscular, dolor de cabeza, dolor de oídos, tos y escalofríos
- Entumecimiento, hormigueo o sensación de escozor en la piel, dolores de manos o pies, mareos, temblor
- Disminución del apetito, cambio en el sabor de las cosas
- Aumento del dolor, tamaño del tumor o enrojecimiento alrededor del tumor.
- Pérdida de peso
- Estreñimiento, diarrea, náuseas, vómitos, dolor de estómago, acidez de estómago
- Niveles bajos de potasio o calcio y/o sodio en la sangre
- Funcionamiento de la tiroides menor del que debería ser
- Dolor de piernas (que podría ser un síntoma de trombosis), dolor de pecho o dificultad respiratoria (que podría ser un síntoma de coágulos de sangre en los pulmones, llamado embolia pulmonar)
- Infecciones de todo tipo, incluidas la infección de los senos paranasales que rodean la nariz (sinusitis), infección del pulmón y de las vías respiratorias altas
- Dificultad respiratoria
- Visión borrosa
- Opacidad del ojo (cataratas)
- Problemas renales que incluyen riñones que no funcionan correctamente o que no son capaces de mantener el funcionamiento normal
- Resultados anómalos en las pruebas hepáticas
- Valores altos en los resultados de las pruebas hepáticas
- Cambios en una proteína de la sangre que puede producir hinchazón de las arterias (vasculitis)
- Aumento de los niveles de azúcar en la sangre (diabetes)
- Disminución de los niveles de azúcar en sangre
- Dolor de cabeza
- Sangrado nasal
- Piel seca
- Depresión, cambios en el estado de ánimo, dificultad para dormir
- Tos
- Bajada de la tensión arterial
- Una sensación vaga de malestar en el cuerpo, sentirse mal
- Inflamación dolorosa de la boca, sequedad de boca
- Deshidratación
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas):
- Destrucción de glóbulos rojos (anemia hemolítica)
- Ciertos tipos de tumores de la piel
- Sangrado de las encías, estómago o intestinos
- Aumento de la tensión, latido cardiaco lento, rápido o irregular
- Aumento de la cantidad de una sustancia que se libera tras la destrucción normal o anormal de los glóbulos rojos
- Aumento de un tipo de proteína que indica inflamación en el organismo
- Oscurecimiento de la piel, cambio de color de la piel como resultado de un sangrado interno, normalmente causado por hematomas, inflamación de la piel causada por la acumulación de sangre, hematoma
- Aumento del ácido úrico en la sangre
- Erupciones cutáneas, enrojecimiento de la piel, piel agrietada, descamación o exfoliación de la piel, urticaria
- Aumento de la sudoración, sudoración nocturna
- Dificultad al tragar, dolor de garganta, dificultad para mantener la calidad de la voz o cambios en la voz
- Goteo nasal
- Fuerte aumento o disminución en la cantidad de orina frente a lo habitual o incapacidad de controlar la micción
- Sangre en la orina
- Dificultad respiratoria especialmente al tumbarse (que podría ser un síntoma de insuficiencia cardiaca)
- Dificultad para tener una erección
- Ictus, desmayo, vértigo (trastorno del oído interno que provoca la sensación de que todo da vueltas), pérdida temporal del conocimiento
- Dolor en el pecho que se extiende a brazos, cuello, mandíbula, espalda o estómago, sensación de sudoración y falta de aire, náuseas o vómitos, que pueden ser síntomas de un ataque al corazón (infarto de miocardio)
- Debilidad muscular, falta de energía
- Dolor cervical, dolor en el pecho
- Escalofríos
- Hinchazón de las articulaciones
- Flujo biliar del hígado más lento o bloqueado
- Niveles bajos de fosfato o magnesio en la sangre
- Dificultad para hablar
- Daño hepático
- Alteración del equilibrio, dificultad de movimientos
- Sordera, pitidos en los oídos (tinnitus)
- Dolor en los nervios, sensación anormal y desagradable, especialmente al tocar
- Exceso de hierro en el organismo
- Sed
- Confusión
- Dolor dental
- Caída que puede causar lesiones
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- Hemorragia en el interior del cráneo
- Problemas circulatorios
- Pérdida de la visión
- Pérdida del deseo sexual (libido)
- Expulsión de grandes cantidades de orina con dolor de huesos y debilidad, que pueden ser síntomas de un trastorno renal (síndrome de Fanconi)
- Pigmentación amarilla en la piel, en las mucosas o en los ojos (ictericia), heces de color pálido, orina de color oscuro, picor de piel, erupción cutánea, dolor o hinchazón del estómago - éstos pueden ser síntomas de daño en el hígado (insuficiencia hepática)
- Dolor de estómago, hinchazón abdominal o diarrea, que pueden ser síntomas de una inflamación del intestino grueso (llamada colitis o tiflitis)
- Daño en las células de los riñones (llamado necrosis tubular renal)
- Cambios en el color de la piel, sensibilidad a la luz solar
- Síndrome de lisis tumoral – se pueden producir complicaciones metabólicas durante el tratamiento del cáncer y algunas veces incluso sin tratamiento. Estas complicaciones se producen como consecuencia de los productos de descomposición de las células tumorales que mueren y pueden incluir: cambios en la bioquímica sanguínea, niveles altos de potasio, fósforo, ácido úrico y niveles bajos de calcio que, por tanto, generan cambios en la función renal y el ritmo cardiaco, crisis convulsivas y, algunas veces, la muerte.
- Aumento de la presión arterial dentro de los vasos sanguíneos que irrigan los pulmones (hipertensión pulmonar).
Efectos adversos de frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- Dolor repentino, o leve que empeora en la parte superior del estómago y/o espalda, que dura varios días, posiblemente acompañado de náuseas, vómitos, fiebre y un pulso rápido. Estos síntomas pueden deberse a una inflamación del páncreas.
- Silbidos o pitidos al respirar, dificultad respiratoria o tos seca, que pueden ser síntomas causados por una inflamación del tejido de los pulmones.
- Se han observado casos raros de degradación muscular (dolor, debilidad o hinchazón muscular) que pueden dar lugar a problemas de riñón (rabdomiólisis), algunos de ellos cuando se administra Lenalidomida Teva con una estatina (un tipo de medicamento para reducir el colesterol).
- Una enfermedad que afecta a la piel producida por la inflamación de los vasos sanguíneos pequeños, acompañada de dolor en las articulaciones y fiebre (vasculitis leucocitoclástica).
- Rotura de la pared del estómago o del intestino. Esto puede dar lugar a una infección muy grave. Informe a su médico si tiene dolor de estómago fuerte, fiebre, náuseas, vómitos, sangre en las heces o cambios en los hábitos intestinales.
- Infecciones virales, que incluyen herpes zóster (también conocido como la “culebrilla”, una enfermedad viral que produce una erupción cutánea dolorosa con ampollas) y la reaparición de la infección por hepatitis B (que puede producir un amarilleamiento de la piel y de los ojos, orina de color marrón oscuro, dolor de estómago en el lado derecho, fiebre y náuseas o sensación de estar enfermo).
- Rechazo de trasplante de órganos sólidos (tales como riñón, corazón).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Lenalidomida Teva
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el blister y la caja después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
No utilice este medicamento si observa indicios visibles de deterioro o signos de manipulación indebida.
Los medicamentos no se deben tirar por los desagües ni a la basura. Devuelva a su farmacéutico el medicamento sin usar. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Lenalidomida Teva
Lenalidomida Teva 5 mg cápsulas duras EFG:
- El principio activo es lenalidomida. Cada cápsula contiene lenalidomida hidrocloruro hidrato correspondiente a 5 mg de lenalidomida.
- Los demás componentes son:
Contenido de la cápsula:
Sílice coloidal anhidra, celulosa microcristalina, croscarmelosa sódica y talco
Cubierta de la cápsula:
Gelatina y dióxido de titanio (E171)
Tinta de impresión:
Goma laca, propilenglicol, óxido de hierro negro (E172), hidróxido de potasio y solución de amoníaco concentrado
Lenalidomida Teva 10 mg cápsulas duras EFG:
- El principio activo es lenalidomida. Cada cápsula contiene lenalidomida hidrocloruro hidrato correspondiente a 10 mg de lenalidomida.
- Los demás componentes son:
Contenido de la cápsula:
Sílice coloidal anhidra, celulosa microcristalina, croscarmelosa sódica y talco
Cubierta de la cápsula:
Gelatina, dióxido de titanio (E171), óxido de hierro amarillo (E172) y carmín índigo (E132)
Tinta de impresión:
Goma laca, propilenglicol, óxido de hierro negro (E172), hidróxido de potasio y solución de amoníaco concentrado
Lenalidomida Teva 15 mg cápsulas duras EFG:
- El principio activo es lenalidomida. Cada cápsula contiene lenalidomida hidrocloruro hidrato correspondiente a 15 mg de lenalidomida.
- Los demás componentes son:
Contenido de la cápsula:
Sílice coloidal anhidra, celulosa microcristalina, croscarmelosa sódica y talco
Cubierta de la cápsula:
Gelatina, dióxido de titanio (E171) y carmín índigo (E132)
Tinta de impresión:
Goma laca, propilenglicol, óxido de hierro negro (E172), hidróxido de potasio y solución de amoníaco concentrado
Lenalidomida Teva 20 mg cápsulas duras EFG:
- El principio activo es lenalidomida. Cada cápsula contiene lenalidomida hidrocloruro hidrato correspondiente a 20 mg de lenalidomida.
- Los demás componentes son:
Contenido de la cápsula:
Sílice coloidal anhidra, celulosa microcristalina, croscarmelosa sódica y talco
Cubierta de la cápsula:
Gelatina, dióxido de titanio (E171), óxido de hierro amarillo (E172) y carmín índigo (E132)
Tinta de impresión:
Goma laca, propilenglicol, óxido de hierro negro (E172), hidróxido de potasio y solución de amoníaco concentrado
Lenalidomida Teva 25 mg cápsulas duras EFG:
- El principio activo es lenalidomida. Cada cápsula contiene lenalidomida hidrocloruro hidrato correspondiente a 25 mg de lenalidomida.
- Los demás componentes son:
Contenido de la cápsula:
Sílice coloidal anhidra, celulosa microcristalina, croscarmelosa sódica y talco
Cubierta de la cápsula:
Gelatina y dióxido de titanio (E171)
Tinta de impresión:
Goma laca, propilenglicol, óxido de hierro negro (E172), hidróxido de potasio y solución de amoníaco concentrado
Aspecto del producto y contenido del envase
Lenalidomida Teva 5 mg cápsulas duras EFG son cápsulas duras, no transparentes, de tamaño “4” (aproximadamente 14,3 mm de longitud), que llevan impreso “5” en negro en el cuerpo blanco y con tapa blanca, conteniendo un polvo de color blanquecino a amarillo pálido o beige o polvo comprimido.
Lenalidomida Teva 10 mg cápsulas duras EFG son cápsulas duras, no transparentes, de tamaño “2” (aproximadamente 18 mm de longitud), que llevan impreso “10” en negro en el cuerpo amarillento y con tapa verde, conteniendo un polvo de color blanquecino a amarillo pálido o beige o polvo comprimido.
Lenalidomida Teva 15 mg cápsulas duras EFG son cápsulas duras, no transparentes, de tamaño “1” (aproximadamente 19,4 mm de longitud), que llevan impreso “15” en negro en el cuerpo blanco y con tapa azul, conteniendo un polvo de color blanquecino a amarillo pálido o beige o polvo comprimido.
Lenalidomida Teva 20 mg cápsulas duras EFG son cápsulas duras, no transparentes, de tamaño “0” (aproximadamente 21,7 mm de longitud), que llevan impreso “20” en negro en el cuerpo azul y con tapa verde, conteniendo un polvo de color blanquecino a amarillo pálido o beige o polvo comprimido.
Lenalidomida Teva 25 mg cápsulas duras EFG son cápsulas duras, no transparentes, de tamaño “0” (aproximadamente 21,7 mm de longitud), que llevan impreso “25” en negro en el cuerpo blanco y con tapa blanca, conteniendo un polvo de color blanquecino a amarillo pálido o beige o polvo comprimido.
Tamaños de envase:
Lenalidomida Teva está disponible en envases blíster que contienen 7, 21 o 63 cápsulas duras y en envases blíster unidosis que contienen 7 x 1, 21 x 1 o 63 x 1 cápsulas duras.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Teva B.V.
Swensweg 5
2031 GA Haarlem
Países Bajos
Responsable de la fabricación
Teva Operations Poland Sp. z.o.o
ul. Mogilska 80
31-546 Kraków
Polonia
ó
Merckle GmbH
Ludwig-Merckle-Strasse 3, Blaubeuren
89143 Baden-Wuerttemberg
Alemania
ó
PLIVA Croatia Ltd.
Prilaz baruna Filipovica 25
10000 Zagreb
Croacia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Teva Pharma, S.L.U.
C/ Anabel Segura, 11. Edificio Albatros B, 1ª planta
28108 Alcobendas, Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo y en Reino Unido (Irlanda del Norte) con los siguientes nombres:
Austria Lenalidomid TEVA 5 mg Hartkapseln
Lenalidomid TEVA 10 mg Hartkapseln
Lenalidomid TEVA 15 mg Hartkapseln
Lenalidomid TEVA 20 mg Hartkapseln
Lenalidomid TEVA 25 mg Hartkapseln
Bélgica Lenalidomide Teva 5 mg harde capsules / gélules / Hartkapseln
Lenalidomide Teva 10 mg harde capsules / gélules / Hartkapseln
Lenalidomide Teva 15 mg harde capsules / gélules / Hartkapseln
Lenalidomide Teva 20 mg harde capsules / gélules / Hartkapseln
Lenalidomide Teva 25 mg harde capsules / gélules / Hartkapseln
República Checa Lenalidomid Teva
Alemania Lenalidomid-ratiopharm 5 mg Hartkapseln
Lenalidomid-ratiopharm 10 mg Hartkapseln
Lenalidomid-ratiopharm 15 mg Hartkapseln
Lenalidomid-ratiopharm 20 mg Hartkapseln
Lenalidomid-ratiopharm 25 mg Hartkapseln
Dinamarca Lenalidomide Teva
Estonia Lenalidomide Teva
España Lenalidomida Teva 5 mg cápsulas duras EFG
Lenalidomida Teva 10 mg cápsulas duras EFG
Lenalidomida Teva 15 mg cápsulas duras EFG
Lenalidomida Teva 20 mg cápsulas duras EFG
Lenalidomida Teva 25 mg cápsulas duras EFG
Finlandia Lenalidomide ratiopharm 5 mg kapseli, kova
Lenalidomide ratiopharm 10 mg kapseli, kova
Lenalidomide ratiopharm 15 mg kapseli, kova
Lenalidomide ratiopharm 20 mg kapseli, kova
Lenalidomide ratiopharm 25 mg kapseli, kova
Francia Lénalidomide Teva 5 mg, gélule
Lénalidomide Teva 10 mg, gélule
Lénalidomide Teva 15 mg, gélule
Lénalidomide Teva 25 mg, gélule
Croacia Lenalidomid Teva 5 mg tvrde kapsule
Lenalidomid Teva 10 mg tvrde kapsule
Lenalidomid Teva 15 mg tvrde kapsule
Lenalidomid Teva 20 mg tvrde kapsule
Lenalidomid Teva 25 mg tvrde kapsule
Hungría Lenalidomid Teva 5 mg kemény kapszula
Lenalidomid Teva 10 mg kemény kapszula
Lenalidomid Teva 15 mg kemény kapszula
Lenalidomid Teva 20 mg kemény kapszula
Lenalidomid Teva 25 mg kemény kapszula
Irlanda Lenalidomide Teva 5 mg Hard Capsules
Lenalidomide Teva 10 mg Hard Capsules
Lenalidomide Teva 15 mg Hard Capsules
Lenalidomide Teva 20 mg Hard Capsules
Lenalidomide Teva 25 mg Hard Capsules
Italia LENALIDOMIDE TEVA
Lituania Lenalidomide Teva 25 mg kietosios kapsules
Letonia Lenalidomide Teva 25 mg cietas kapsulas
Luxemburgo Lenalidomide Teva 5 mg gélules dures
Lenalidomide Teva 10 mg gélules dures
Lenalidomide Teva 15 mg gélules dures
Lenalidomide Teva 20 mg gélules dures
Lenalidomide Teva 25 mg gélules dures
Malta Lenalidomide Teva 10 mg Hard Capsules
Lenalidomide Teva 15 mg Hard Capsules
Lenalidomide Teva 25 mg Hard Capsules
Países Bajos Lenalidomide Teva 5 mg, harde capsules
Lenalidomide Teva 10 mg, harde capsules
Lenalidomide Teva 15 mg, harde capsules
Lenalidomide Teva 20 mg, harde capsules
Lenalidomide Teva 25 mg, harde capsules
Noruega Lenalidomide Teva
Portugal Lenalidomide Teva
Suecia Lenalidomide Teva
Eslovenia Lenalidomid Teva 5 mg trde kapsule
Lenalidomid Teva 10 mg trde kapsule
Lenalidomid Teva 15 mg trde kapsule
Lenalidomid Teva 20 mg trde kapsule
Lenalidomid Teva 25 mg trde kapsule
Eslovaquia Lenalidomide Teva B.V. 5 mg
Lenalidomide Teva B.V. 10 mg
Lenalidomide Teva B.V. 15 mg
Lenalidomide Teva B.V. 25 mg
Islandia Lenalidomide Teva
Reino Unido Lenalidomide 5 mg, 10 mg, 15 mg, 20 mg, 25 mg Hard Capsules
(Irlanda del Norte)
Fecha de la última revisión de esteprospecto: Junio 2023
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/)
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LENALIDOMIDA TEVA 20 MG CAPSULAS DURAS EFGForma farmacéutica: CAPSULA, 10 mgPrincipio activo: LenalidomidaFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: CAPSULA, 15 mgPrincipio activo: LenalidomidaFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: CAPSULA, 2,5 mgPrincipio activo: LenalidomidaFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para LENALIDOMIDA TEVA 20 MG CAPSULAS DURAS EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LENALIDOMIDA TEVA 20 MG CAPSULAS DURAS EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






