
LAINEMA 14/3 g/100 ml SOLUCION RECTAL EFG


Cómo usar LAINEMA 14/3 g/100 ml SOLUCION RECTAL EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
LAINEMA 14/3 g/100 ml solución rectal EFG
Dihidrógenofosfato de sodio (monohidratado)/Hidrógenofosfato de disodio (dodecahidratado)
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Contenido del prospecto:
- Qué es LAINEMA 14/3 g/100 ml solución rectal EFG y para qué se utiliza
- Antes de usar LAINEMA 14/3 g/100 ml solución rectal EFG
- Cómo usar LAINEMA 14/3 g/100 ml solución rectal EFG
- Posibles efectos adversos
- Conservación de LAINEMA 14/3 g/100 ml solución rectal EFG
- Información adicional
1. Qué es LAINEMA 14/3 g/100 ml solución rectal EFG y para qué se utiliza
Este medicamento pertenece al grupo farmacoterapéutico de los laxantes de administración rectal.
Este medicamento está indicado en aquellos casos en que sea necesaria una evacuación intestinal, tales como: en pre y post-cirugía, parto y post-parto, antes de rectoscopia, sigmoidoscopia y colonoscopia (técnicas de exploración del intestino grueso), antes de exámenes radiológicos, impactación fecal (acúmulo de heces endurecidas en el recto).
2. ANTES DE USAR LAINEMA 14/3 g/100 ml solución rectal EFG
No use LAINEMA 14/3 g/100 ml solución rectal EFG
- si es alérgico (hipersensible) a los principios activos o a cualquiera de los demás componentes de este medicamento
- en niños menores de 2 años
- si existe sospecha de oclusión intestinal (imposibilidad de eliminación de heces y gases)
- si presenta megacolon (dilatación del colon), ileostomía (ano artificial), estenosis anorrectal (estrechamiento del recto), ano imperforado (ausencia u obstrucción congénita del orificio anal) o íleo paralítico (parálisis del intestino)
- si presenta problemas de riñón graves o moderados
- si presenta insuficiencia cardiaca congestiva (problema grave del corazón)
- si presenta síntomas de apendicitis o perforación intestinal
- si presenta hemorragia rectal sin diagnosticar
- si padece hipertensión arterial (tensión alta) no controlada
- si padece deshidratación y, en general, en todos los casos donde la distribución del contenido de LAINEMA en el organismo pueda estar aumentada o su eliminación disminuida.
En caso de duda sobre cualquiera de esas situaciones o de la suya en particular consulte con su médico.
Tenga especial cuidado con LAINEMA 14/3 g/100 ml solución rectal EFG
Si después de 10 minutos de la administración del enema no consigue evacuar (la evacuación se produce aproximadamente 5 minutos después de la administración), se recomienda consultar con su médico para que le realice las pruebas necesarias para minimizar el riesgo de aparición de cuadros graves de hiperfosfatemia.
- Si es anciano, se encuentra débil, sufre ascitis (acumulación de líquido en el abdomen), úlceras o fisuras anales o padece alguna enfermedad de los riñones o del corazón.
- Si le han practicado una colostomía (cirugía mayor del colon) debe consultar con su médico.
- Si tiene desequilibrios electrolíticos preexistentes (alteración de los niveles de sales minerales en el organismo) ya que puede aparecer hipocalcemia (niveles disminuidos de calcio en sangre), hipopotasemia (niveles disminuidos de potasio en sangre), hiperfosfatemia (niveles aumentados de fósforo en sangre), hipernatremia (niveles aumentados de sodio en sangre) y acidosis.
- En caso de sospecha de trastornos electrolíticos su médico le realizará un análisis de sangre para determinar los niveles de estas sustancias, antes de la administración de LAINEMA.
- Si tiene usted náuseas, vómitos o dolor abdominal debe seguir las indicaciones de su médico.
- No se recomienda el uso repetido y prolongado de LAINEMA, ya que puede producir habituación. No utilizar si los síntomas empeoran o persisten sin consultar al médico.
- LAINEMA debe ser administrado siguiendo las instrucciones de uso y manipulación. Deberá interrumpir la administración si se encuentra resistencia ya que si fuerza su administración puede provocarse lesiones.
- Si después de la administración del enema sangra por el recto, no se administre más enema y consulte inmediatamente con el médico.
- Consultar al médico si se producen signos de irritación.
Este medicamento no debe utilizarse como tratamiento habitual del estreñimiento.
Uso de otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta médica, homeopáticos, plantas medicinales y otros productos relacionados con la salud, ya que puede ser necesario interrumpir el tratamiento o ajustar la dosis de alguno de ellos.
Es especialmente importante que su médico sepa si está tomando medicamentos para el tratamiento de la tensión alta o angina de pecho (bloqueantes del canal del calcio), medicamentos para vaciar la vejiga (diuréticos), medicamentos para ciertos trastornos de la conducta (litio) u otros fármacos que pudieran modificar el equilibrio del agua o de los electrólitos (minerales) en la sangre.
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
No utilice este medicamento durante el embarazo o en periodo de lactancia sin consultar al médico.
Si se encuentra en periodo de lactancia deberá extraerse y desechar la leche que produzca durante las 24 horas siguientes a la administración de LAINEMA.
Conducción y uso de máquinas
Este medicamento no afecta su capacidad para conducir o utilizar máquinas.
Información importante sobre algunos de los componentes de LAINEMA 14/3 g/100 ml solución
rectal EFG
Puede producir reacciones alérgicas (posiblemente retardadas) y, excepcionalmente, broncoespasmo, porque contiene parahidroxibenzoato de metilo, sal de sodio (E-219).
3. Cómo usar LAINEMA 14/3 g/100 ml solución rectal EFG
Siga exactamente las instrucciones de administración del medicamento indicadas por su médico. Consulte a su médico o farmacéutico si tiene dudas.
Éste es un medicamento de administración rectal. Se debe aplicar a temperatura ambiente, sin necesidad de calentar.
Para la auto-aplicación de este medicamento se recomienda que el paciente esté reclinado sobre el lado izquierdo y con ambas piernas dobladas sobre el pecho (Figura 1) o reclinado con la pierna izquierda extendida y la derecha doblada sobre el pecho (Figura 2).

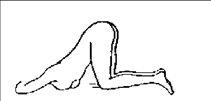
Cuando este medicamento vaya a ser administrado al paciente por otra persona, las posiciones recomendadas pueden ser o bien las descritas para la auto-aplicación, o la que aparece en la Figura 3.

Presionar hacia un lado el capuchón. Así, se rompe el precinto. Una vez roto el precinto, extraer hacia arriba el capuchón y la cánula prelubrificada ya queda libre para inserción.
En las posiciones indicadas, introdúzcase la cánula en el recto, de forma cuidadosa para evitar lesionar la pared del mismo, y oprímase el envase, de manera suave y continuada, hasta que penetre la cantidad de líquido requerida. Conviene que el paciente mantenga dicha posición hasta que sienta fuertes deseos de defecar. Generalmente, 2 a 5 minutos son suficientes para obtener el efecto deseado. Si no se expulsa el producto al cabo de este tiempo, ver el apartado 4: “Posibles efectos adversos”.
En general, se recomienda la siguiente dosis:
Niños
-Lactantes y niños menores de 2 años: LAINEMA no debe administrarse en lactantes y niños menores de 2 años, su uso está contraindicado.
-Niños de 2 a 15 años:La dosis recomendada es de una dosis única de 5 ml/kg, o hasta un máximo de 140 ml. La duración máxima del tratamiento será de un enema al día, durante no más de 6 días consecutivos.
Adultos
La dosis recomendada es de un único enema de 140 ml ó 250 ml. Se podrá administrar una vez al día, durante un máximo de 6 días consecutivos.
Mayores de 65 años: La pauta posológica recomendada es la misma que para adultos.
Pacientes con problemas de hígado: En este caso, no es necesario ajuste de dosis.
Pacientes con problemas de riñón:No debe administrarse este medicamento a pacientes con problemas
de riñón graves o moderados.
Se administrará con precaución a pacientes con problemas de riñón leves y sólo bajo prescripción médica.
Si usa más LAINEMA 14/3 g/100 ml solución rectal EFG del que debiera
En caso de sobredosis o de ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar LAINEMA 14/3 g/100 ml solución rectal EFG
No use una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, LAINEMA puede producir efectos adversos, aunque no todas las personas los sufran.
En muy raros casos, pueden darse casos de tetania (espasmos dolorosos de los músculos de las extremidades) con hipocalcemia (disminución del calcio en la sangre) e hiperfosfatemia (aumento del fósforo en la sangre) graves. Se han notificado casos graves de hiperfosfatemia asociada a la administración de laxantes con alto contenido en fosfatos. Los pacientes con factores de riesgo para desarrollar hiperfosfatemia deberán, por lo tanto, ser controlados por medio de pruebas analíticas (ver apartado “Tenga especial cuidado con LAINEMA 14/3 g/100 ml solución rectal EFG”)
Los pacientes que desarrollen de forma muy rara hiperfosfatemia grave pueden presentar irritabilidad, hipotensión (presión arterial baja), calambres musculares, cianosis (coloración azulada de la piel), tetania, taquicardia (aumento del ritmo del corazón), convulsiones, obnubilación, cansancio, debilidad o, de forma potencial, un estado comatoso.
A continuación se describen los efectos adversos conocidos de LAINEMA y se enumeran según la frecuencia con la que se presenten:
Efectos adversos muy raros(que afectan a menos de 1 de cada 10.000 pacientes):
-tetania
-hipocalcemia
-hiperfosfatemia grave
-ampollas
-escozor
-picor
-irritación rectal
-dolor
Efectos adversos muy frecuentes(que afectan al menos a 1 de cada 10 pacientes)
- Hiperfosfatemia transitoria
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
5. Conservación de LAINEMA 14/3 g/100 ml solución rectal EFG
No requiere condiciones especiales de conservación, no obstante en raras ocasiones pueden aparecer algunas floculaciones inorgánicas en la solución que de ninguna manera afectan a la integridad del preparado.
Mantener fuera del alcance y de la vista de los niños.
No utilice LAINEMA después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. INFORMACIÓN ADICIONAL
Composición de LAINEMA 14/3 g/100 ml solución rectal EFG
Los principios activos por cada mililitro son: dihidrógenofosfato de sodio (monohidratado) 139 mg, hidrógenofosfato de disodio (dodecahidratado) 32 mg. Los demás componentes (excipientes) son: parahidroxibenzoato de metilo, sal de sodio (E-219) y agua purificada.
Aspecto del producto y contenido del envase
Este medicamento pertenece a un grupo de medicamentos llamados laxantes de administración rectal.
Se presenta en forma de solución rectal, en frascos de polietileno de baja densidad de 80, 140 y 250 ml y aplicador rectal formado por tapón de polietileno de alta densidad, cánula de EBA (copolímero de etileno con acrilato de butilo) ya lubrificada, precinto y tapón de polietileno de alta densidad..
Titular de la autorización de comercialización y responsable de la fabricación
LAINCO, S.A. Avda. Bizet, 8-12 - 08191 Rubí (Barcelona)
Este prospecto ha sido aprobado en Octubre 2017
- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LAINEMA 14/3 g/100 ml SOLUCION RECTAL EFGForma farmacéutica: LIQUIDO RECTAL, 3,2 g / 13,9 gPrincipio activo: Fosfato disodioFabricante: Casen Recordati S.L.Requiere recetaForma farmacéutica: LIQUIDO RECTAL, 6,14 ml glicerolPrincipio activo: GlicerolFabricante: Casen Recordati S.L.No requiere recetaForma farmacéutica: LIQUIDO RECTAL, 450 mg / 45 mgPrincipio activo: sodium lauryl sulfoacetate, incl. combinationsFabricante: Lainco S.A.No requiere receta
Médicos online para LAINEMA 14/3 g/100 ml SOLUCION RECTAL EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LAINEMA 14/3 g/100 ml SOLUCION RECTAL EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















