
LAGROBEN 5 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS

Cómo usar LAGROBEN 5 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para elusuario
Lagroben 5 mg/ml colirio en solución en envase unidosis
Carmelosa sódica
Lea todo el prospecto detenidamente antes de empezar ausar estemedicamento, porque contiene información importante para usted.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si necesita consejo o más información, consulte a su farmacéutico.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Debe consultar a un médico si empeora o si no mejora después de 3 días.
Contenido del prospecto
- Qué es Lagroben y para qué se utiliza
- Qué necesita saber antes de empezar a usar Lagroben
- Cómo usar Lagroben
- Posibles efectos adversos
- Conservación de Lagroben
- Contenido del envase e información adicional
1. Qué es Lagroben y para qué se utiliza
Lagroben es un sustituto de lágrimas y contiene un lubricante llamado carmelosa sódica. Este medicamento se utiliza para el alivio sintomático de la irritación y la sequedad ocular.
Debe consultar a un médico si empeora o si no mejora después de 3 días.
2. Que necesita saber antes de empezar a usar Lagroben
No use Lagroben
Si es alérgico a la carmelosa sódica o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Si se produce irritación, dolor, enrojecimiento o cambios en la visión o si considera que su situación empeora, deje de usar este medicamento y consulte a su médico o farmacéutico.
Uso de Lagroben con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado o podría tener que utilizar cualquier otro medicamento.
Embarazoylactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Lagroben puede utilizarse durante el embarazo y la lactancia.
Conducción y uso de máquinas
Lagroben puede provocar visión borrosa de corta duración, normalmente de 1 a 15 minutos. En caso de experimentar visión borrosa temporal, no conduzca ni utilice máquinas hasta que su visión sea clara.
3. Cómo usar Lagroben
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico. En caso de duda, pregunte a su médico o farmacéutico.
Lagroben se utiliza por vía oftálmica (se aplica en el ojo).
La dosis recomendada es una gota en el ojo u ojos afectados tantas veces como sea necesario o según le indique su especialista.
Asegúrese de que el envase unidosis está intacto antes del uso. La solución debe usarse inmediatamente después de su apertura. Para evitar una posible contaminación del cuentagotas y de la solución, el gotero no debe entrar en contacto con el ojo o con cualquier otra superficie.
Lávese las manos antes de su uso.
Para una correcta administración, siga las siguientes instrucciones:
- Desprender una unidad de la tira de envases unidosis.
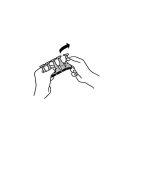
- Compruebe que el envase unidosis no está dañado.
- Sujete el envase unidosis entre los dedos índice y pulgar de una mano.
Con los dedos índice y pulgar de la otra mano, efectúe una ligera palanca sobre la pieza alada para su apertura. No girar para romper.
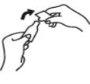
- Retire la pieza alada.

- Tire suavemente hacia abajo del párpado inferior para formar una bolsa. Gire el envase unidosis hacia abajo y apriete éste para aplicar una gota en cada ojo. Parpadee un par de veces.

No use de nuevo el envase unidosis aunque quede solución restante.
Si se emplea más de un medicamento por vía oftálmica, las aplicaciones de los distintos medicamentos deben espaciarse al menos 5 minutos.
Si usa más Lagroben de la que debiera
No le producirá ningún daño. Si está preocupado, hable con su médico o farmacéutico.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, Teléfono 91 562 04 20, indicando el medicamento y la cantidad administrada o ingerida.
Si olvidó usar Lagroben
Aplique la siguiente dosis según sea necesario, o siguiendo la pauta normal que le ha indicado su médico o farmacéutico. No use una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Lagroben puede producir efectos adversos, aunque no todas las personas los sufran.
Se han comunicado los siguientes efectos adversos, pero el número de personas que pueden verse afectadas es desconocido:
- Irritación del ojo, sensación de quemazón o escozor,
- Visión borrosa,
- Aumento de la producción de lágrimas (también conocido como lagrimeo).
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Lagroben
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar los envases unidosis en el embalaje original.
Desechar el envase unidosis abierto después de usar (no reutilizar el envase una vez abierto para las siguientes dosis).
No utilice este medicamento si observa que la solución cambia de color o se enturbia.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto Sigre de la farmacia. En caso de dudapregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Lagroben:
- El principio activo es carmelosa sódica. Cada ml de solución contiene 5 mg de carmelosa sódica.
- Los demás componentes son cloruro de sodio, lactato de sodio, cloruro de potasio, cloruro de calcio dihidrato, cloruro de magnesio hexahidrato, hidróxido de sodio, ácido clorhídrico y agua para preparaciones inyectables.
Los componentes de Lagroben han sido diseñados para simular la composición natural de su lágrima.
Aspecto del producto y contenido del envase
Lagroben se presenta en envases unidosis de 0,4 ml de solución estéril. Cada estuche de cartón contiene una bolsa de aluminio con 10 o 30 envases unidosis.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Teva Pharma, S.L.U.
C/ Anabel Segura, 11. Ed. Albatros B, 1ª planta
28108 Alcobendas - Madrid
Responsable de la fabricación
Neuraxpharm Pharmaceuticals, S.L.Avda. de Barcelona, 69
08970 Sant Joan Despi (Barcelona), España
Fecha de la última revisión de este prospecto:Febrero2014
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es /
- País de registro
- Principio activo
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LAGROBEN 5 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSISForma farmacéutica: COLIRIO, 5,5 mg sodio cloruro;3 mg hipomelosa/mlPrincipio activo: artificial tears and other indifferent preparationsFabricante: Alcon Healthcare S.A.No requiere recetaForma farmacéutica: COLIRIO, 3,2 mg/mlPrincipio activo: artificial tears and other indifferent preparationsFabricante: Bausch & Lomb S.A.No requiere recetaForma farmacéutica: COLIRIO, 3,2 mg/mlPrincipio activo: artificial tears and other indifferent preparationsFabricante: Bausch & Lomb S.A.No requiere receta
Médicos online para LAGROBEN 5 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LAGROBEN 5 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















