
KREON 35.000 U CAPSULAS DURAS GASTRORRESISTENTES

Cómo usar KREON 35.000 U CAPSULAS DURAS GASTRORRESISTENTES
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
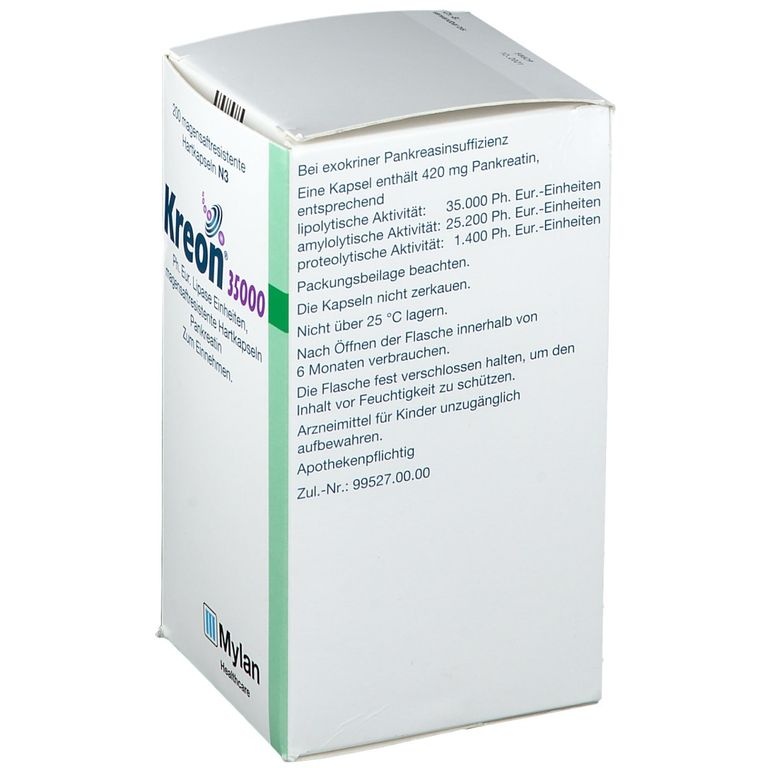
Kreon 35.000 U cápsulas duras gastrorresistentes
Polvo de páncreas
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4
- Qué es Kreon y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Kreon
- Cómo tomar Kreon
- Posibles efectos adversos
- Conservación de Kreon
- Contenido del envase e información adicional
1. Qué es Kreon y para qué se utiliza
Qué es Kreon
- Kreon contiene una combinación de enzimas denominada “polvo de páncreas”.
- El polvo de páncreas también se denomina pancreatina. Ayuda a digerir los alimentos. Las enzimas se extraen de las glándulas del páncreas de los cerdos.
- Las cápsulas de Kreon contienen pequeños gránulos que liberan el polvo de páncreas de forma lenta en su intestino (gránulos gastroresistentes, denominados minimicroesferas).
Para qué se utiliza Kreon
Kreon se utiliza para el tratamiento de la “insuficiencia pancreática exocrina”. Se trata de un trastorno en el que la glándula del páncreas no produce suficientes enzimas para digerir los alimentos. Se produce con frecuencia, por ejemplo, en personas que:
- Padecen fibrosis quística, un raro desorden genético.
- Sufren inflamación crónica del páncreas (pancreatitis crónica).
- Se les ha extirpado todo o parte del páncreas (pancreatectomía total o parcial).
- Padecen cáncer pancreático.
Kreon 35.000 se puede utilizar en niños, adolescentes y adultos. La dosificación por grupos de edad se explica en la sección 3 de este prospecto, “Cómo tomar Kreon”.
El tratamiento con Kreon mejora los síntomas de la insuficiencia pancreática exocrina, incluida la consistencia de las heces (p. ej., heces con grasa), el dolor abdominal, la flatulencia y la frecuencia de las deposiciones (diarrea o estreñimiento), independientemente de la enfermedad subyacente.
Cómo funciona Kreon
Las enzimas de Kreon funcionan digiriendo los alimentos cuando pasan por el intestino. Debe tomar Kreon durante o inmediatamente después de una comida o un refrigerio. Esto permitirá que las enzimas se mezclen bien con la comida.
2. . Qué necesita saber antes de empezar a tomar Kreon
No tome Kreon
- Si es alérgico al polvo de páncreas o a cualquiera de los demás componentes de Kreon (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Kreon.
Pacientes con fibrosis quística
Se ha detectado una rara dolencia intestinal, denominada “colonopatía fibrosa”, en la que se estrecha el intestino, en pacientes con fibrosis quística que toman productos con altas dosis de polvo de páncreas. Si tiene fibrosis quística y toma más de 10.000 unidades de lipasa por kilogramo y día y experimenta síntomas abdominales inusuales o cambios en los síntomas abdominales, comuníqueselo a su médico.
La dosificación de unidades de lipasa se explica en la sección 3 de este prospecto, “Cómo tomar Kreon”.
Reacción alérgica grave
Si se produce una reacción alérgica, pare el tratamiento y consulte a su médico. Una reacción alérgica puede incluir picor, urticaria o erupciones. Rara vez, una reacción alérgica más seria puede incluir sensación de calor, mareos y desmayos, problemas al respirar; son síntomas de una afección grave y potencialmente mortal denominada “shock anafiláctico”. Si ocurre, solicite inmediatamente atención médica urgente.
Consulte a su médico si es alérgico a las proteínas del cerdo antes de tomar Kreon.
Irritación de boca
Puede producirse dolor bucal, irritación (estomatitis), sangrado y formación de úlceras en la boca en caso de que se mastiquen las cápsulas o se mantengan demasiado tiempo en la boca. Enjuagar la boca y beber un vaso de agua puede ayudar si hay signos preliminares de irritación bucal.
Kreon solo se puede espolvorear en determinados alimentos (ver sección 3 de este prospecto, “Cómo tomar Kreon”).
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por unidad de dosis, por lo que se considera esencialmente exento de sodio.
Uso de Kreon con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que pudiera quedarse embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Su médico decidirá si puede tomar Kreon durante el embarazo.
Kreon se puede utilizar durante la lactancia materna.
Conducción y uso de máquinas
Kreon no afecta a su capacidad para conducir o utilizar herramientas o maquinaria.
3. Cómo tomar Kreon
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, pregunte a su médico o farmacéutico.
Su dosis se mide en “unidades de lipasa”. La lipasa es una de las enzimas del polvo de páncreas. Las distintas concentraciones de Kreon contienen cantidades distintas de lipasa.
Siga siempre el consejo médico acerca de qué dosis de Kreon tomar. Su médico ajustará la dosis a sus necesidades.
Dependerá de:
- la gravedad de su enfermedad
- su peso
- su dieta
- cuánta grasa hay en sus deposiciones
Si tiene deposiciones grasas u otros problemas estomacales o intestinales (síntomas gastrointestinales), consulte a su médico, ya que es posible que sea necesario ajustar la dosis.
Cuánto Kreon tomar
Para pacientes con fibrosis quística
Niños:
La concentración de este medicamento puede no ser adecuada para la iniciación del tratamiento en niños, según su edad y peso.
El médico debe determinar la dosis necesaria para un niño con formas de dosificación que contengan menos unidades de lipasa (p. ej., 10.000 o 5.000 unidades de lipasa).
Una vez establecida la dosis por comida, esta concentración del medicamento se puede utilizar en niños.
- La dosis inicial normal para niños menores de 4 años es de 1000 unidades de lipasa por kilogramo de peso corporal y comida.
- La dosis inicial normal para niños a partir de 4 años es de 500 unidades de lipasa por kilogramo de peso corporal y comida.
Adolescentes y adultos:
La dosificación de la enzima basada en el peso debe comenzar con 500 unidades de lipasa por kilogramo de peso corporal por comida en adolescentes y adultos.
Para todos los grupos de edad:
La dosis no debe superar las 2.500 unidades de lipasa por kilogramo de peso corporal por comida ni las 10.000 unidades de lipasa por kilogramo de peso corporal por día ni las 4000 unidades de lipasa por gramo de grasa ingerido.
Para pacientes con otros problemas de páncreas
Adolescentes y adultos:
La dosis habitual para una comida se encuentra entre 25.000 y 80.000 unidades de lipasa.
La dosis habitual para un refrigerio es la mitad de dosis que para una comida.
Cuándo tomar Kreon
Tome siempre Kreon durante o inmediatamente después de una comida o un refrigerio. Esto permitirá que las enzimas se mezclen bien con la comida y que se digiera cuando pase por el intestino.
Cómo tomar Kreon
- Kreon debe tomarse siempre con una comida o un refrigerio.
- Las cápsulas se deben tragar enteras con un vaso de agua o zumo.
- No rompa ni mastique las cápsulas ni su contenido, ya que puede ocasionar irritación en el interior de la boca o cambiar la forma en que Kreon trabaja en su organismo.
- Si le resulta difícil tragar las cápsulas, ábralas con cuidado y añada los gránulos a una pequeña cantidad de comida ácida suave o líquidos ácidos. Comidas ácidas suaves pudieran ser, por ejemplo, yogur o compota de manzana. Los líquidos ácidos pudieran ser zumo de manzana, naranja o piña. No mezcle los gránulos con agua, leche, incluida la leche saborizada, leche materna o leche de fórmula, ni con comida caliente. Ingiera la mezcla inmediatamente, sin triturar ni masticar y beba agua o zumo.
- Mezclar con comida o líquidos no ácidos, triturar o masticar los gránulos puede causar irritación en el interior de la boca o cambiar la forma en que Kreon trabaja en su organismo.
- No mantenga las cápsulas de Kreon o su contenido en el interior de la boca. Asegúrese de que el medicamento y la mezcla de comida se ingieren por completo y que no quedan gránulos en el interior de la boca.
- No almacene la mezcla.
Si toma más Kreon del que debe
Si toma más Kreon del que debe, beba mucha agua y consulte con su médico o farmacéutico.
En ocasiones, dosis muy altas de polvo de páncreas han ocasionado demasiado ácido úrico en la orina (hiperuricosuria) y en la sangre (hiperuricemia).
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91.562.04.20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Kreon
Si olvida una dosis, tome la siguiente dosis a la hora habitual, con su siguiente comida. No tome una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento con Kreon
No deje de tomar Kreon sin antes comunicárselo a su médico. Muchos pacientes tendrán que tomar Kreon durante el resto de su vida.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Kreon puede producir efectos adversos, aunque no todas las personas los sufran.
Con este medicamento se pueden producir los siguientes efectos secundarios.
Los efectos secundarios más importantes observados con los medicamentos de sustitución de la enzima pancreática son “shock anafiláctico” y colonopatía fibrosa. Estos dos efectos secundarios se han producido en un número muy reducido de personas, pero se desconoce su frecuencia exacta.
El shock anafiláctico es una reacción alérgica grave y potencialmente mortal que se puede desarrollar rápidamente.
Si experimenta alguno de los siguientes síntomas, busque atención médica inmediata:
- Picor, urticaria o erupciones.
- Hinchazón de cara, ojos, labios, manos o pies.
- Debilidad o desmayo.
- Dificultad para respirar o tragar.
- Palpitaciones.
- mareo, colapso o inconsciencia.
Las altas dosis repetidas de medicamentos de sustitución de la enzima pancreática también pueden ocasionar cicatrización o engrosamiento de la pared intestinal que puede provocar el bloqueo de los intestinos, una enfermedad denominada colonopatía fibrosa. Si tiene dolor de estómago intenso, problemas de flujo intestinal (estreñimiento), náuseas o vómitos, consulte con su médico inmediatamente.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- Dolor de estómago (abdomen).
Efectos adversos frecuentes(pueden afectar hasta a 1 de cada 10 personas)
- Sensación de malestar (náuseas).
- Malestar (vómitos).
- Estreñimiento.
- Hinchazón (distensión abdominal).
- Diarrea.
Pueden deberse a la condición para la que está tomando Kreon.
Efectos adversos poco frecuentes(pueden afectar hasta a 1 de cada 100 personas)
- Erupción.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Kreon
Mantenga este medicamento fuera del alcance y de la vista de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase o blíster. La fecha de caducidad es el último día del mes que se indica.
No debe conservarse a temperatura superior a 25 °C.
Una vez abierto, no debe almacenarse por encima de 25 °C y debe utilizarse antes de que transcurran 6 meses. Mantenga el envase perfectamente cerrado para protegerlo de la humedad.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Kreon
El ingrediente activo en Kreon es el polvo de páncreas.
- Cada cápsula de Kreon 35.000 contiene 420 mg de polvo de páncreas que se corresponden a (unidades de farmacopea europea):
| 35.000 |
| 25.200 |
| 1.400 |
- Los demás componentes son:
Contenido de la cápsula:
- Ftalato de hipromelosa
- Macrogol 4000
- Citrato de trietilo
- Dimeticona 1000
- Alcohol cetílico
Cubierta de la cápsula:
- Gelatina
- Óxidos de hierro rojo y amarillo(E 172)
- Laurilsulfato sódico
Aspecto del producto y contenido del envase
Las cápsulas de Kreon 35.000 son de talla 00 elongada. Son de color naranja y transparente. Contienen gránulos gastroresistentes de color parduzco (minimicroesferas).
Kreon 35.000 está disponible en frascos de HDPE con tapones de polipropileno roscados 50 cápsulas, 60 cápsulas, 100 cápsulas, 120 cápsulas y 200 cápsulas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart, Dublín 15
Dublín
Irlanda
Responsable de la fabricación:
Abbott Laboratories GmbH
Justus-von-Liebig-Str. 33
31535 Neustadt
Alemania
o
Mylan Germany GmbH
Lütticher Straße 5
53842 Troisdorf
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Viatris Pharmaceuticals, S.L.
C/ General Aranaz, 86
28027 Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria | KREON 35.000 Einheiten - Kapseln |
Bélgica | Creon 35.000, 300 mg, maagsapresistente capsules, hard |
Bulgaria | Kreon 35.000 gastro-resistant capsules, hard |
Croacia | KREON® 35.000 želucanootporne kapsule, tvrde |
Chipre | Creon 35.000 |
República checa | KREON |
Dinamarca | Creon 35.000 |
Estonia | Kreon 35.000 U |
Finlandia | Creon 35.000 enterokapseli, kova |
Francia | CREON 35.000 U, gélule gastro-résistante |
Grecia | Creon-P 35.000 gastro-resistant capsule, hard |
Hungría | Kreon 35.000 egység gyomornedv-ellenálló kemény kapszula |
Islandia | Creon 35.000 sýruþolin hylki, hörð |
Irlanda | Creon 35.000 Gastro-resistant Capsules |
Italia | CreonIPE 35.000 |
Letonia | Kreon 35.000 V zarnas škistošas cietas kapsulas |
Lituania | Kreon 35.000 V skrandyje neirios kietosios kapsules |
Luxemburgo | Creon 35.000, 300 mg, gélules gastro-résistantes |
Malta | Creon® 35.000 Capsules |
Países bajos | Creon 35.000, maagsapresistente capsules |
Noruega | Creon 35.000 |
Polonia | Kreon 35.000 |
Portugal | KREON 35.000, Cápsula gastro-resistente |
Rumanía | KREON 35.000 capsule gastrorezistente |
Eslovaquia | Kreon 35.000, tvrdé gastrorezistentné kapsuly |
Eslovenia | Uprašeni pankreas Mylan 35.000 Ph.Eur.e. trde gastrorezistentne kapsule |
España | Kreon 35.000 U cápsulas duras gastrorresistentes |
Suecia | Creon 35.000 enterokapslar, hårda |
Reino Unido | Creon® 35.000 Capsules |
Fecha de la última revisión de este prospecto: Septiembre 2018
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a KREON 35.000 U CAPSULAS DURAS GASTRORRESISTENTESForma farmacéutica: CAPSULA, -Principio activo: multienzymes (lipase, protease etc.)Fabricante: Viatris Healthcare LimitedRequiere recetaForma farmacéutica: CAPSULA, -Principio activo: multienzymes (lipase, protease etc.)Fabricante: Viatris Healthcare LimitedRequiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 100 mg de pellets (minimicroesferas) gastrorresistPrincipio activo: multienzymes (lipase, protease etc.)Fabricante: Viatris Healthcare LimitedRequiere receta
Médicos online para KREON 35.000 U CAPSULAS DURAS GASTRORRESISTENTES
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de KREON 35.000 U CAPSULAS DURAS GASTRORRESISTENTES, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















