JIVI 3000 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar JIVI 3000 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para elusuario
Jivi 250 UI polvo y disolvente para solucióninyectable
Jivi 500 UI polvo y disolvente para solucióninyectable
Jivi 1000 UI polvo y disolvente para solucióninyectable
Jivi 2000 UI polvo y disolvente para solucióninyectable
Jivi 3000 UI polvo y disolvente para solucióninyectable
factor VIII de coagulación humano recombinante pegilado con dominio B suprimido (damoctocog alfa pegol)
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Jivi y para qué se utiliza
- Qué necesita saber antes de empezar a usar Jivi
- Cómo usar Jivi
- Posibles efectos adversos
- Conservación de Jivi
- Contenido del envase e información adicional
1. Qué es Jivi y para qué se utiliza
Jivi contiene el principio activo damoctocog alfa pegol. Se produce con tecnología recombinante sin adición de ningún componente de origen humano o animal en el proceso de fabricación. El factor VIII es una proteína que se encuentra de forma natural en la sangre y ayuda a que coagule. La proteína de damoctocog alfa pegol ha sido modificada (pegilada) para prolongar su acción en el organismo.
Jivi se utiliza para tratar y prevenir la hemorragiaen adultos y adolescentes de 12 años o mayores con hemofilia A (déficit hereditario de factor VIII) previamente tratados. No es para uso en niños menores de 12 años.
2. Qué necesita saber antes de empezar a usar Jivi
No use Jivi si es:
- alérgico a damoctocog alfa pegol o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- alérgico a las proteínas de ratón o hámster.
Advertencias y precauciones
Consulte a su médico o farmacéutico si:
? presenta opresión en el pecho, una caída de la presión arterial (que se manifiesta a menudo por una sensación de mareo cuando se levanta rápidamente), urticaria con picor, sibilancias (silbidos al respirar), malestar general o desmayo. Estos pueden ser signos de una reacción alérgica repentinagrave rara a este medicamento. Interrumpainmediatamente la inyección del medicamentoy consiga de inmediato asistencia médica si esto ocurre.
- tiene una hemorragia que no se llega a controlar con su dosis habitual de este medicamento. Hable con su médico de inmediato si esto ocurre. Es posible que haya desarrollado anticuerpos frente al factor VIII (inhibidores) o anticuerpos frente al polietilenglicol (PEG). Estos anticuerpos reducen la eficacia de Jivi en la prevención y el control de las hemorragias. Su médico le puede realizar análisis para confirmar esta posibilidad y asegurarse de que su dosis de Jivi genera niveles adecuados de factor VIII. Si es necesario, su médico le puede cambiar otra vez al tratamiento con factor VIII que usaba previamente.
- ha desarrollado con anterioridad inhibidores del factor VIII con otro producto diferente.
- padece una enfermedad cardíaca o tiene riesgo de padecer una enfermedad cardíaca.
- tiene que utilizar un dispositivo de acceso venoso central para este medicamento. Puede tener riesgo de sufrir complicaciones relacionadas con el dispositivo en la zona donde se inserta el catéter, que incluyen:
- infecciones locales
- presencia de bacterias en la sangre
- formación de un coágulo de sangre en el vaso sanguíneo
Niños
Jivi no se puede usar en niños menores de 12 años.
Otros medicamentos y Jivi
Se desconoce si Jivi afecta o puede verse afectado por otros medicamentos. Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
La influencia de Jivi sobre la capacidad para conducir y utilizar máquinas es nula.
Jivi contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Jivi
El tratamiento con Jivi se iniciará por un médico con experiencia en el cuidado de pacientes con hemofilia A. Después de recibir la formación pertinente, los pacientes o cuidadores pueden ser capaces de administrar Jivi en su casa. Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda consulte de nuevo a su médico o farmacéutico. La dosis de unidades del factor VIII se mide en Unidades Internacionales (UI).
Tratamiento de la hemorragia
Para tratar la hemorragia, su médico calculará y ajustará su dosis y con qué frecuencia se debe administrar, dependiendo de factores, tales como:
- su peso
- la gravedad de su hemofilia A
- la localización y gravedad de la hemorragia
- si tiene inhibidores del factor VIII y cómo de altos son esos niveles
- el nivel requerido de factor VIII
Prevención de la hemorragia
Para prevenir la hemorragia, su médico seleccionará una dosis y una frecuencia adecuadas en función de sus necesidades:
- 45-60 UI por kg de peso corporal cada 5 días o
- 60 UI por kg de peso corporal cada 7 días o
- 30-40 UI por kg de peso corporal dos veces por semana.
Pruebas de laboratorio
Las pruebas analíticas a intervalos apropiados ayudan a comprobar que tenga siempre niveles adecuados de factor VIII. En el caso concreto de las intervenciones de cirugía mayor, se debe controlar de cerca su coagulación sanguínea.
Duración del tratamiento
Generalmente, el tratamiento para la hemofilia con Jivi será necesario de por vida.
Cómo se administra Jivi
Jivi se inyecta en una vena durante 2-5 minutos dependiendo del volumen total y de su grado de comodidad. La velocidad máxima es de 2,5 ml por minuto. Jivi se debe usar en el plazo de 3 horas después de su reconstitución.
Cómo se prepara Jivi para la inyección
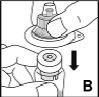
Utilice únicamente los componentes (el adaptador de vial, la jeringa precargada con el disolvente y el equipo para punción venosa) incluidos en el envase para este medicamento. Consulte a su médico si no es posible utilizar estos componentes. Si alguno de los componentes del envase está abierto o dañado, no utilice este componente.
El medicamento reconstituido se debe filtrar utilizando el adaptador de vialantes de la inyección para eliminar posibles partículas presentes en la solución.
Este medicamento nose debe mezclar con otras inyecciones. No utilice soluciones que estén turbias o si contienen partículas visibles. Siga las instrucciones de usoque le dé su médico y las que figuran al final de este prospecto.
Si usa más Jivi del que debe
Informe a su médico si esto ocurre. No se han comunicado síntomas de sobredosis.
Si olvidó usar Jivi
Inyecte inmediatamente la siguiente dosis y continúe a intervalos regulares siguiendo las indicaciones de su médico.
No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Jivi
No deje de usar este medicamento sin consultarlo con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos más gravesson reacciones alérgicaso una reacción alérgica grave. Interrumpa la inyección de Jivi inmediatamente y hable enseguida con su médico si se produce una reacción de este tipo.Los siguientes síntomas podrían ser un signo precoz de estas reacciones:
- opresión en el pecho o sensación general de malestar
- ardor y picor en la zona de aplicación
- urticaria, sofocos
- disminución de la presión arterial que puede dar sensación de mareo al levantarse
- náuseas
En los pacientes que han recibido un tratamiento previo con factor VIII (más de 150 días de tratamiento), se podrían formar anticuerpos inhibidores (ver sección 2) en casos raros (menos de 1 de cada 100 pacientes). Si esto ocurre, su medicamento podría dejar de funcionar bien y podría experimentar hemorragia persistente. Si esto ocurre, se debe poner en contacto con su médico inmediatamente.
Con este medicamento pueden ocurrir los siguientes efectos adversos:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- dolor de cabeza
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- dolor de estómago
- náuseas, vómitos
- fiebre
- reacciones alérgicas (se pueden presentar en forma de ronchas, urticaria generalizada, opresión en el pecho, sibilancias (silbidos al respirar), falta de aliento, disminución de la presión arterial; para síntomas iniciales, ver más arriba)
? reacciones locales en la zona de inyección, como hemorragia debajo de la piel, picor fuerte, hinchazón, sensación de quemazón, enrojecimiento pasajero
- mareo
- dificultad para conciliar el sueño
- tos
- sarpullido, enrojecimiento de la piel
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- inhibición del factor VIII
- alteración del gusto
- sofoco
- picor
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos
directamente a través del Sistema Español de Farmacovigilancia de Medicamanetos de Uso Humano www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Jivi
Mantener este medicamento fuera de la vista y del alcance de los niños.
Noutilice este medicamento después de la fecha de caducidad que aparece en las etiquetas y en las cajas. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC). Nocongelar.
Conservar el vial y la jeringa precargada en el embalaje exterior para protegerlo de la luz.
Este medicamento se puede conservar a temperatura ambiente (inferior a 25 °C) durante un máximo de 6 meses, si lo mantiene en el envase. Si se conserva a temperatura ambiente, éste caduca después de 6 meses o en la fecha de caducidad indicada, lo que suceda antes.
Debe anotar la nueva fecha de caducidad en el embalaje exterior cuando extraiga el medicamento de la nevera.
Norefrigerar la solución tras la reconstitución. La solución reconstituida se debe utilizar en un plazo máximo de 3 horas.
Noutilice este medicamento si observa partículas o si la solución está turbia.
Este medicamento es para un solo uso. Desechar la solución no utilizada.
Los medicamentos nose deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico o médico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Jivi
- El principio activo es el factor VIII de coagulación humano recombinante pegilado con dominio B suprimido (damoctocog alfa pegol). Cada vial de Jivi contiene 250 ó 500 ó 1000 ó 2000 ó 3000 UI de damoctocog alfa pegol. Tras la reconstitución con el disolvente suministrado (agua estéril para preparaciones inyectables), las soluciones preparadas tienen la siguiente concentración:
Dosis | Concentración aproximada tras la reconstitución |
250 UI | (100 UI / ml) |
500 UI | (200 UI / ml) |
1 000 UI | (400 UI / ml) |
2 000 UI | (800 UI / ml) |
3 000 UI | (1 200 UI / ml) |
- Los demás componentes son sacarosa, histidina, glicina, cloruro de sodio (ver sección 2 “Jivi contiene sodio”), cloruro de calcio dihidrato, polisorbato 80, ácido acético glacial y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Jivi se presenta en forma de polvo y disolvente para solución inyectable. El polvo es seco y de blanco a ligeramente amarillo. El disolvente es un líquido transparente. Tras la reconstitución la solución es transparente.
Cada envase de Jivi contiene
- un vial de vidrio con polvo
- una jeringa precargada con disolvente
- una varilla de émbolo independiente
- un adaptador de vial
- un equipo para punción venosa
Jivi está disponible en envases de:
- 1 envase individual
- 1 envase múltiple con 30 envases individuales
Puede que no todos los tamaños estén comercializados.
Titular de la autorización de comercialización
Bayer AG
51368 Leverkusen
Alemania
Responsable de la fabricación
Bayer AG
Kaiser-Wilhelm-Allee
51368 Leverkusen
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Bayer SA-NV Tél/Tel: +32-(0)2-535 63 11 | Lietuva UAB Bayer Tel. +37 05 23 36 868 |
???????? ????? ???????? ???? T??.: +359-(0)2 4247280 | Luxembourg/Luxemburg Bayer SA-NV Tél/Tel: +32-(0)2-535 63 11 |
Ceská republika Bayer s.r.o. Tel: +420 266 101 111 | Magyarország Bayer Hungária KFT Tel:+36 14 87-41 00 |
Danmark Bayer A/S Tlf: +45 45 23 50 00 | Malta Alfred Gera and Sons Ltd. Tel: +35 621 44 62 05 |
Deutschland Bayer Vital GmbH Tel: +49 (0)214-30 513 48 | Nederland Bayer B.V. Tel: +31-23-799 1000 |
Eesti Bayer OÜ Tel: +372 655 8565 | Norge Bayer AS Tlf: +47 23 13 05 00 |
Ελλ?δα Bayer Ελλ?ς ΑΒΕΕ Τηλ: +30-210-61 87 500 | Österreich Bayer Austria Ges.m.b.H. Tel: +43-(0)1-711 46-0 |
España Bayer Hispania S.L. Tel: +34-93-495 65 00 | Polska Bayer Sp. z o.o. Tel: +48 22 572 35 00 |
France Bayer HealthCare Tél (N° vert): +33-(0)800 87 54 54 | Portugal Bayer Portugal, Lda. Tel: +351 21 416 42 00 |
Hrvatska Bayer d.o.o. Tel: +385-(0)1-6599 900 | România SC Bayer SRL Tel: +40 21 529 59 00 |
Ireland Bayer Limited Tel: +353 1 216 3300 | Slovenija Bayer d. o. o. Tel: +386 (0)1 58 14 400 |
Ísland Icepharma hf. Sími: +354 540 8 000 | Slovenská republika Bayer spol. s r.o. Tel. +421 2 59 21 31 11 |
Italia Bayer S.p.A. Tel: +39 02 397 8 1 | Suomi/Finland Bayer Oy Puh/Tel: +358- 20 785 21 |
Κ?προς NOVAGEM Limited Tηλ: +357 22 48 38 58 | Sverige Bayer AB Tel: +46 (0) 8 580 223 00 |
Latvija SIA Bayer Tel: +371 67 84 55 63 | United Kingdom Bayer plc Tel: +44-(0)118 206 3 000 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
------------------------------------------------------------------------------------------------------------------
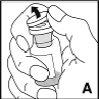
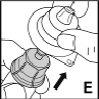
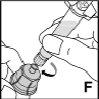
Instrucciones detalladas de reconstitución y administración de Jivi
Necesitará gasas estériles impregnadas en alcohol, gasas estériles, apósitos y un torniquete. Estos artículos no están incluidos en el envase de Jivi.
| |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
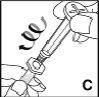
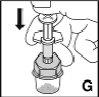
Compruebe que todo el contenido del vial ha pasado a la jeringa. Mantenga la jeringa en posición vertical y presione el émbolo hasta que no quede aire en la jeringa. |
|
| |
| |
| |
|
|
| |
| |
| |
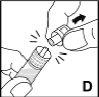
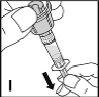
Finalmente, aplique con una gasa una pequeña presión sobre la zona de inyección y si es necesario ponga un apósito. | |
| |
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a JIVI 3000 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 1.000 UIPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 1.500 UIPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 1000 UI - tras reconstitución en 2 ml de agua para inyectables la dosis es de 500 UI/mlPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere receta
Médicos online para JIVI 3000 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de JIVI 3000 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes