JAYDESS 13,5 MG SISTEMA DE LIBERACION INTRAUTERINO


Cómo usar JAYDESS 13,5 MG SISTEMA DE LIBERACION INTRAUTERINO
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para la usuaria
Jaydess 13,5mg sistema de liberación intrauterino
levonorgestrel
?Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Jaydess y para qué se utiliza
- Qué necesita saber antes de empezar a usar Jaydess
- Cómo usar Jaydess
- Posibles efectos adversos
- Conservación de Jaydess
- Contenido del envase e información adicional
1. Qué es Jaydess y para qué se utiliza
Jaydess se utiliza para la prevención del embarazo (anticoncepción) con una duración de hasta tres años.
Jaydess es un sistema de liberación intrauterino (SLI) en forma de T que, tras su colocación dentro del útero, libera lentamente una pequeña cantidad de la hormona levonorgestrel.
Jaydess funciona reduciendo el crecimiento mensual del revestimiento del útero y espesando el moco cervical. Estas acciones impiden que el esperma y el óvulo entren en contacto e impiden así la fecundación de un óvulo por el esperma.
2. Qué necesita saber antes de empezar a usar Jaydess
Consideraciones generalesAntes de empezar a usar Jaydess, su médico le hará algunas preguntas sobre sus antecedentes clínicos personales. En este prospecto se describen varias situaciones en las que se debería extraer Jaydess, o en las que la fiabilidad de Jaydess puede disminuir. En dichas situaciones, usted no debería mantener relaciones o debería utilizar un preservativo u otro método de barrera. Jaydess, al igual que otros anticonceptivos hormonales, no protege frente a la infección por VIH (SIDA) ni frente a ninguna otra enfermedad de transmisión sexual. Jaydess no es adecuado para su empleo como anticonceptivo de emergencia (anticonceptivo postcoital). |
NO use Jaydess
- si está embarazada (ver sección "Embarazo, lactancia y fertilidad")
- si padece actualmente una enfermedad inflamatoria pélvica (EIP; infección de los órganos reproductores femeninos) o ha padecido esta afección varias veces en el pasado
- si padece afecciones asociadas a una mayor propensión a las infecciones pélvicas
- si padece una infección del tracto genital inferior (una infección de la vagina o del cuello uterino)
- si ha padecido una infección del útero después de dar a luz o después de un aborto provocado o espontáneo durante los últimos 3 meses
- si actualmente tiene anomalías celulares en el cuello uterino
- si padece o sospecha que padece cáncer del cuello uterino o del útero
- si tiene tumores que son sensibles a las hormonas progestagénicas para crecer, p.ej. cáncer de mama
- si padece sangrado uterino por causa no conocida
- si padece una anomalía del cuello uterino o del útero, incluyendo fibromas que deforman la cavidad del útero
- si padece una enfermedad hepática o tumor hepático activos
- si es alérgica al levonorgestrel o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Antes de utilizar Jaydess informe a su médico si:
- padece diabetes. Por lo general no hay necesidad de modificar su medicación antidiabética mientras utiliza Jaydess, pero tal vez su médico deba comprobarlo
- padece epilepsia. Se puede producir un ataque (crisis) durante la colocación o la extracción
- ha tenido en el pasado un embarazo ectópico o extrauterino (embarazo fuera del útero).
Asimismo, hable también con su médico si presenta cualquiera de las situaciones que se detallan a continuación antes de utilizar Jaydess o si alguna de ellas aparece por primera vez mientras utiliza este medicamento:
- migraña, con alteraciones visuales u otros síntomas que puedan ser signos de una isquemia cerebral transitoria (obstrucción temporal del suministro de sangre al cerebro)
- dolor de cabeza excepcionalmente intenso
- ictericia (coloración amarillenta de la piel, el blanco de los ojos y/o las uñas)
- marcado incremento de la tensión arterial
- enfermedades arteriales graves tales como ictus o ataque al corazón.
Los siguientes signos y síntomas podrían significar que usted puede tener un embarazo extrauterino y debe consultar a su médico inmediatamente (ver también sección "Embarazo, lactancia y fertilidad"):
- sus períodos menstruales han cesado y después empieza a tener un sangrado persistente o dolor
- tiene un dolor intenso o persistente en la parte baja del abdomen
- tiene signos normales de embarazo, pero también tiene sangrado y se siente mareada
- tiene una prueba de embarazo positiva.
Póngase en contacto con su médico inmediatamente si se produce cualquiera de las siguientes situaciones (ver también sección 4) y recuerde informarle que tiene insertado Jaydess, especialmente si no es la persona que se lo insertó:
- dolor grave (como calambres menstruales) o sangrado abundante después de la colocación o si experimenta dolor/sangrado que se prolonga durante más de unas semanas. Esto puede ser, por ejemplo, un signo de infección, de perforación o de que Jaydess no está en su posición correcta.
- ya no nota los hilos en su vagina. Esto puede ser un signo de expulsión o perforación. Puede comprobarlo introduciendo con cuidado un dedo en su vagina y notando los hilos al final de su vagina, cerca de la abertura del útero (cuello uterino). No tire de los hilos, ya que accidentalmente puede extraer Jaydess. Use un método de barrera (como los preservativos) hasta que su médico haya comprobado que el SLI está aún en su posición.
- usted o su pareja pueden sentir la parte inferior de Jaydess. Evite las relaciones sexuales hasta que su médico haya comprobado que el SLI está aún en su posición.
- su pareja nota los hilos de extracción durante la relación sexual.
- piensa que puede estar embarazada.
- presenta dolor abdominal persistente, fiebre o un flujo anormal de la vagina, que puede ser un signo de infección. Las infecciones deben ser tratadas inmediatamente.
- siente dolor o molestias durante las relaciones sexuales, lo que puede ser, por ejemplo, un signo de infección, de quiste ovárico o de que Jaydess no está en su posición correcta.
- se producen cambios repentinos en sus periodos menstruales (por ejemplo, si presenta un sangrado menstrual escaso o nulo y después empieza a experimentar un sangrado o dolor persistentes, o empieza a sangrar abundantemente), lo que puede ser un signo de que Jaydess no está en su posición correcta o se ha expulsado.
Se recomienda el uso de compresas. Si se usan tampones o copas menstruales, debe cambiarlos con cuidado para no tirar de los hilos de Jaydess. Si cree que puede haber movido Jaydess de su posición (consulte la lista anterior para conocer los posibles signos), evite las relaciones sexuales o use un método de barrera (como los preservativos), y póngase en contacto con su médico.
Trastornos psiquiátricos
Algunas mujeres que utilizan anticonceptivos hormonales como Jaydess han notificado depresión o un estado de ánimo deprimido. La depresión puede ser grave y a veces puede inducir pensamientos suicidas.
Si experimenta alteraciones del estado de ánimo y síntomas depresivos, póngase en contacto con su médico para obtener asesoramiento médico adicional lo antes posible.
Niños y adolescentes
Jaydess no está indicado para su uso antes del primer sangrado menstrual (menarquia).
Otros medicamentos y Jaydess
Informe a su médico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Embarazo
Jaydess no se debe utilizar durante el embarazo.
En algunas mujeres puede desaparecer el periodo mientras usan Jaydess. No tener el periodo no es necesariamente un signo de embarazo. Si usted no tiene el periodo y tiene otros síntomas de embarazo debe acudir a su médico para que le haga un reconocimiento y una prueba de embarazo.
Si no ha tenido la regla durante seis semanas y está preocupada, considere hacerse una prueba de embarazo. Si es negativa, no hace falta realizar otra prueba a menos que tenga otros signos de embarazo.
Si se queda embarazada con Jaydess colocado, debe acudir a su profesional sanitario inmediatamente para extraer Jaydess. La extracción puede provocar un aborto. Sin embargo, si se deja Jaydess en su ubicación durante el embarazo, no sólo es mayor el riesgo de sufrir un aborto espontáneo, si no también el de tener un parto prematuro. Si no se puede extraer Jaydess, consulte con su profesional sanitario sobre los beneficios y los riesgos de continuar con el embarazo. Si el embarazo continúa, se le monitorizará estrechamente durante el mismo y deberá ponerse en contacto con su profesional sanitario de inmediato si experimenta calambres en el estómago, dolor de estómago o fiebre.
Jaydess contiene una hormona, llamada levonorgestrel, y se han notificado casos aislados de efectos en los genitales de bebés de sexo femenino si se exponen a dispositivos intrauterinos de levonorgestrel mientras están el útero.
Si desea quedarse embarazada, debe ponerse en contacto con su médico para que le extraiga Jaydess.
Embarazo extrauterino (embarazo fuera del útero)
Es poco frecuente quedarse embarazada mientras utiliza Jaydess. No obstante, si se queda embarazada mientras utiliza Jaydess, aumenta el riesgo de que el embarazo se produzca fuera del útero (de que tenga un embarazo extrauterino o ectópico). Las mujeres que ya han tenido un embarazo extrauterino, una intervención quirúrgica de las trompas de Falopio o una infección pélvica corren mayor riesgo de este tipo de embarazo. Un embarazo extrauterino es un cuadro grave que exige asistencia médica inmediata (ver sección 2, "Advertencias y precauciones" para los signos y síntomas) y puede afectar a la fertilidad futura.
Lactancia
Puede utilizar Jaydess durante la lactancia. Se ha identificado levonorgestrel (el principio activo de Jaydess) en pequeñas cantidades en la leche materna de mujeres lactantes. No obstante, no se han observado efectos negativos sobre el crecimiento y desarrollo del bebé ni sobre la cantidad o calidad de la leche materna.
Fertilidad
Su nivel habitual de fertilidad retornará después de extraer Jaydess.
Conducción y uso de máquinas
La influencia de Jaydess sobre la capacidad para conducir y utilizar máquinas es nula.
3. Cómo usar Jaydess
- Antes de insertar Jaydess debe asegurarse de no estar embarazada.
- Jaydess debe insertarse en el plazo de 7 días desde el inicio de la menstruación. Cuando se inserta durante esos días, Jaydess funciona desde la inserción y evitará que se quede embarazada.
- Si no le pueden insertar Jaydess en el plazo de 7 días desde el inicio de la menstruación o si su menstruación no es regular, entonces se puede insertar Jaydess cualquier otro día. En este caso, no debe haber mantenido relaciones sexuales sin métodos anticonceptivos desde su último periodo menstrual, así como debe tener un test de embarazo negativo antes de la inserción. Además, es posible que Jaydess no prevenga de forma fiable el embarazo desde el momento de la inserción. Por tanto, debe utilizar un método anticonceptivo de barrera (como el preservativo) o debe abstenerse de mantener relaciones sexuales vaginales en los siguientes 7 días después de la inserción de Jaydess.
- Jaydess no está indicado para su empleo como anticonceptivo de emergencia (anticonceptivo postcoital).
Empezando a utilizar Jaydess después de dar a luz
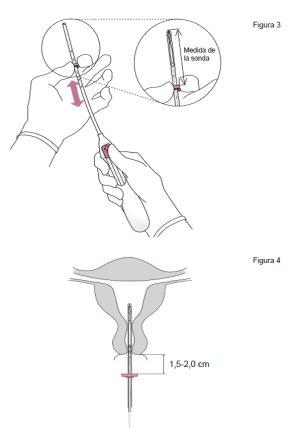
- Se puede insertar Jaydess tras dar a luz después de que el útero haya recuperado el tamaño normal, y no antes de 6 semanas después del parto (ver sección 4 “Posibles efectos adversos – Perforación”).
- Ver también “Empezando a utilizar Jaydess” arriba para saber qué mas se necesita saber para el momento de la inserción.
Empezando a utilizar Jaydess después de un aborto
Se puede insertar Jaydess inmediatamente después de un aborto si el embarazo ha durado menos de 3 meses, siempre que no haya infecciones genitales. Jaydess funcionará desde el momento de la inserción.
Sustitución de Jaydess
Jaydess se puede sustituir en cualquier momento de su ciclo menstrual por otro nuevo Jaydess. Jaydess funcionará desde el momento de la inserción.
Cambio desde otro método anticonceptivo (como anticonceptivos hormonales combinados, implantes)
- Jaydess se puede insertar inmediatamente si se puede asegurar que usted no está embarazada.
- Si han pasado más de 7 días desde el inicio del sangrado menstrual, debe abstenerse de mantener relaciones sexuales vaginales o debe utilizar protección anticonceptiva adicional los siguientes 7 días.
Inserción de Jaydess
Los reconocimientos por parte de su médico antes de la colocación pueden incluir:
- una citología del cuello uterino (prueba de Papanicolaou)
- exploración de las mamas
- otras pruebas, p. ej. para excluir infecciones, entre ellas las enfermedades de transmisión sexual, test de embarazo, si es necesario. Su médico también realizará un reconocimiento ginecológico para determinar la posición y tamaño del útero.
Después de un reconocimiento ginecológico:
- se introduce un instrumento llamado espéculo en la vagina y el cuello uterino puede ser limpiado con una solución antiséptica. Se coloca después Jaydess en el útero utilizando un tubo de plástico estrecho y flexible (el tubo de inserción). Se puede aplicar anestesia local en el cuello uterino antes de la colocación.
- algunas mujeres experimentan mareo o desmayos durante la colocación o después de colocar o extraer Jaydess.
- puede experimentar algo de dolor y sangrado durante la colocación o poco después.
Después de la colocación de Jaydess, su médico debe proporcionarle una tarjeta recordatorio para la paciente para las visitas de seguimiento. Lleve esta tarjeta en cada visita.
Visita de seguimiento y cuándo consultar con su médico:
Debería hacerse revisar Jaydess 4–6 semanas después de su colocación, y posteriormente de forma periódica, al menos una vez al año. Su médico determinará la frecuencia y el tipo de revisiones que se adaptan a su caso individual. Lleve la tarjeta recordatorio para la paciente que le ha proporcionado su médico a cada visita. Además, debe contactar con su médico si aparece alguno de los síntomas descritos en la sección 2 “Advertencias y precauciones”.
Extracción de Jaydess
Jaydess debe extraerse a más tardar al final del tercer año de uso.
Su médico puede extraer Jaydess fácilmente en cualquier momento, tras lo cual el embarazo es posible. Algunas mujeres pueden sentirse mareadas o desmayarse durante o después de la extracción de Jaydess. Puede experimentar algo de dolor y sangrado durante la extracción de Jaydess.
Continuación de la anticoncepción después de la extracción
Si no desea quedarse embarazada, Jaydess no se debe extraer después del séptimo día del ciclo menstrual (período mensual), a no ser que utilice otros métodos de anticoncepción (p. ej. preservativos) durante al menos 7 días antes de la extracción del SLI.
Si tiene periodos irregulares (menstruación) o no tiene el periodo, debe utilizar un método anticonceptivo de barrera durante 7 días antes de la extracción.
También puede insertarse un nuevo Jaydess inmediatamente después de la extracción, en cuyo caso no hace falta protección adicional. Si no desea continuar con el mismo método anticonceptivo, pida consejo a su médico sobre otros métodos anticonceptivos fiables.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Jaydess puede producir efectos adversos, aunque no todas las personas los sufran.
Contacte con su médico inmediatamente si nota cualquiera de estos síntomas:
- reacciones alérgicas, entre ellas sarpullido, ronchas (urticaria) y angioedema (caracterizado por hinchazón repentina de los ojos, la boca y la garganta, por ejemplo).
Consulte también la sección 2 para saber cuándo ponerse en contacto con su médico de inmediato.
Se presenta a continuación una lista de posibles efectos adversos en función de su frecuencia:
Efectos adversos muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- dolor de cabeza
- dolor abdominal/pélvico
- acné/piel grasa
- cambios menstruales, entre ellos aumento o disminución del sangrado menstrual, manchado, períodos infrecuentes y ausencia de sangrado (ver también la sección siguiente sobre el sangrado irregular e infrecuente)
- quiste ovárico (ver también la sección siguiente sobre los quistes ováricos)
- inflamación de los órganos genitales externos y de la vagina (vulvovaginitis)
Efectos adversos frecuentes:pueden afectar hasta 1 de cada 10 personas
- estado de ánimo deprimido/depresión
- disminución de la libido
- migraña
- ganas de vomitar (náuseas)
- pérdida del pelo
- infección del tracto genital superior
- menstruación dolorosa
- dolor/molestias en las mamas
- expulsión del dispositivo (completa y parcial); (ver sección siguiente sobre la expulsión)
- flujo genital
- aumento de peso
Efectos adversos poco frecuentes:pueden afectar hasta 1 de cada 100 personas
- mareos
- exceso de vello corporal
- perforación del útero (ver también la siguiente sección sobre perforación)
Descripción de posibles efectos adversos seleccionados:
Sangrado irregular o infrecuente
Es probable que Jaydess afecte a su ciclo menstrual. Puede modificar sus períodos menstruales de forma que tenga manchado (una pequeña cantidad de sangrado), períodos más largos o más cortos, sangrado más o menos abundante, o ausencia total de sangrado.
Puede experimentar sangrado y manchado entre períodos menstruales, especialmente durante los primeros 3 a 6 meses. A veces el sangrado es más abundante de lo habitual al principio.
En general, es probable que experimente una reducción gradual de la cantidad y del número de días de sangrado cada mes. Algunas mujeres finalmente se encuentran con que la regla se interrumpe por completo.
Puede ser que no se produzca el engrosamiento mensual del revestimiento del útero debido al efecto de la hormona y por tanto no hay nada que pueda salir o desprenderse en forma de período menstrual. Esto no significa necesariamente que haya alcanzado la menopausia o que esté embarazada. Sus propios niveles hormonales suelen permanecer normales.
Cuando se extrae el sistema, el período debe volver pronto a la normalidad.
Infección pélvica
El insertor de Jaydess y el propio Jaydess son estériles. A pesar de ello, existe mayor riesgo de infección pélvica (infecciones del revestimiento del útero o de las trompas de Falopio) en el momento de la colocación y durante las 3 primeras semanas tras la colocación.
Las infecciones pélvicas en las usuarias de SLI suelen estar relacionadas con la presencia de enfermedades de transmisión sexual. El riesgo de infección aumenta si usted o su pareja tienen varias parejas sexuales o si ha padecido una enfermedad inflamatoria pélvica (EIP) anteriormente.
Las infecciones pélvicas deben tratarse inmediatamente.
Las infecciones pélvicas como la EIP pueden tener consecuencias graves y pueden alterar la fertilidad e incrementar el riesgo de un futuro embarazo extrauterino (embarazo fuera del útero). En casos extremadamente raros puede ocurrir una infección grave o septicemia (infección muy grave que puede tener un desenlace mortal) poco después de la inserción.
Jaydess debe extraerse si experimenta EIP recurrente o si una infección es grave o no responde al tratamiento.
Expulsión
Las contracciones musculares del útero durante la menstruación pueden a veces empujar el SLI fuera de su sitio o expulsarlo. Es más probable que esto ocurra si tiene sobrepeso en el momento de la inserción del SLI o si tiene antecedentes de menstruaciones abundantes. Si el SLI se sale de su sitio, es posible que no funcione como es debido y, por tanto, el riesgo de embarazo aumenta. Si el SLI se expulsa, ya no está protegida frente al embarazo.
Los síntomas posibles de una expulsión son dolor y sangrado anormal pero, Jaydess también puede ser expulsado sin darse cuenta. Debido a que Jaydess reduce el flujo menstrual, un aumento del mismo puede ser indicativo de una expulsión.
Se recomienda que usted verifique los hilos con su dedo, por ejemplo, mientras se ducha. Vea también la sección 2, "Advertencias y precauciones" para conocer cómo comprobar si Jaydess está en su sitio. Si presentase signos que indiquen la expulsión o no fuera capaz de palpar los hilos, debería usar un método anticonceptivo adicional (como preservativo), y consultar con su profesional sanitario.
Perforación
Durante la colocación de Jaydess se puede producir una penetración o perforación de la pared del útero, aunque la perforación puede no detectarse hasta más adelante. Si Jaydess se aloja fuera de la cavidad del útero, no es efectivo para prevenir el embarazo y se debe extraer tan pronto como sea posible. Puede necesitar una intervención quirúrgica para extraer Jaydess. El riesgo de perforación aumenta en mujeres en periodo de lactancia así como en mujeres que han dado a luz hasta 36 semanas antes de la inserción y puede aumentar en mujeres con el útero fijo inclinado hacia atrás (útero en retroversión y fijo). Si sospecha que puede haber sufrido una perforación, busque asistencia médica rápidamente y recuérdele que tiene insertado Jaydess, especialmente si no fue la persona que se lo insertó.
Quiste ovárico
Como el efecto anticonceptivo de Jaydess se debe principalmente a su efecto local en el útero, la ovulación (liberación del óvulo) suele continuar mientras se utiliza este medicamento. A veces se puede formar un quiste ovárico. En la mayoría de los casos no hay síntomas.
Un quiste ovárico puede requerir asistencia médica, o más raramente una intervención quirúrgica, pero normalmente desaparece por sí solo.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Jaydess
No requiere condiciones especiales de conservación.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No abra el blíster. Solo su médico o enfermero deben hacerlo.
No utilice este medicamento después de la fecha de caducidad que aparece en el estuche y en el blíster después de "CAD". La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Jaydess
El principio activoes el levonorgestrel. El sistema de liberación intrauterino contiene 13,5 mg de levonorgestrel.
Los demás componentesson:
- elastómero de polidimetilsiloxano
- sílice coloidal anhidra
- polietileno
- sulfato de bario
- óxido de hierro negro (E172)
- plata
Aspecto del producto y contenido del envase
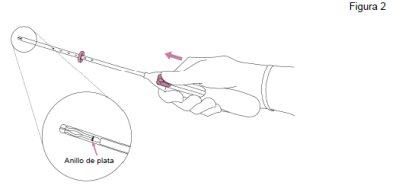
Jaydess es un sistema de liberación intrauterino (SLI) en forma de T. El brazo vertical del cuerpo en forma de T lleva un depósito de fármaco que contiene levonorgestrel. Hay dos hilos de extracción atados a un asa en el extremo inferior del brazo vertical. Además, el eje vertical tiene un anillo de plata situado cerca de los brazos horizontales, que es visible por exploración ecográfica.
Tamaño de envase:
- 1 x 1 sistema de liberación intrauterino.
- 5 x 1 sistema de liberación intrauterino.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Bayer Hispania, S.L.
Av. Baix Llobregat 3-5
08970 Sant Joan Despí (Barcelona)
España
Responsable de la fabricación
Bayer Oy
Pansiontie 47
20210 Turku
Finlandia
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo y en el Reino Unido (Irlanda del Norte) con los siguientes nombres:
- Austria, Bélgica, República Checa, Dinamarca, Finlandia, Francia, Alemania, Hungría, Islandia, Irlanda, Italia, Luxemburgo, Malta, Noruega, Portugal, Rumanía, España, Suecia: Jaydess
- Estonia, Letonia, Lituania: Fleree
Fecha de la última revisión de este prospecto: Mayo 2024
Otras fuentes de información
Puede acceder a información detallada y actualizada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el prospecto, cartonaje y tarjeta recordatorio para la paciente. También puede acceder a la misma información en la siguiente dirección de internet: https://cima.aemps.es/info/77169
---------------------------------------------------------------------------------------------------------------------------
La siguiente información está destinada únicamente a profesionales sanitarios:
INSTRUCCIONES DE INSERCIÓN
Jaydess 13,5 mg sistema de liberación intrauterino
Para inserción por un profesional sanitario empleando una técnica aséptica.
Jaydess se suministra dentro de un insertor en un envase estéril que no debe abrirse hasta que sea necesario para su inserción. No reesterilizar. En esta presentación, Jaydess es para un solo uso. No utilizar si el blíster está dañado o abierto. No insertar después de la fecha de caducidad que aparece en el estuche y en el blíster después de "CAD".
La eliminación del medicamento no utilizado o del material de desecho se realizará de acuerdo con la normativa local.
Jaydess se proporciona con una tarjeta recordatorio para la paciente dentro del envase. Complete la tarjeta y désela a la paciente después de la inserción.
Preparación para la inserción
- Examinar a la paciente para descartar contraindicaciones para la inserción de Jaydess (ver Ficha Técnica, sección 4.3 y sección 4.4 bajo Exploración/consulta médica).
- Insertar un espéculo, visualizar el cuello uterino y después limpiar meticulosamente el cuello uterino y la vagina con una solución antiséptica adecuada.
- Asistirse por un ayudante si es necesario.
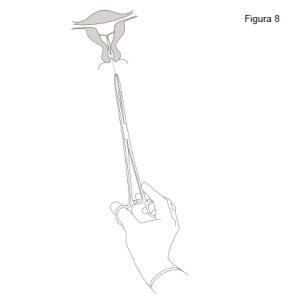
- Sujetar el labio anterior del cuello uterino con un tenáculo u otras pinzas para estabilizar el útero. Si el útero está retrovertido, puede resultar más apropiado sujetar el labio posterior del cuello uterino. Se puede aplicar una tracción suave con las pinzas para enderezar el canal cervical. Las pinzas deben permanecer en su sitio y hay que aplicar una tracción contraria suave sobre el cuello uterino durante toda la intervención de inserción.
- Introducir una sonda uterina por el canal cervical hasta el fondo uterino para medir la profundidad y confirmar la dirección de la cavidad uterina y para descartar cualquier posibilidad de anomalía intrauterina (p. ej., tabique, fibromas submucosos) o de presencia de un anticonceptivo intrauterino insertado anteriormente que no haya sido extraído. Si se encuentran dificultades, considerar la dilatación del canal. Si es necesaria una dilatación cervical, valorar la utilización de analgésicos y/o de un bloqueo paracervical.
Inserción
- Primero, abrir el envase estéril por completo. Después emplear una técnica aséptica y guantes estériles.
|
|
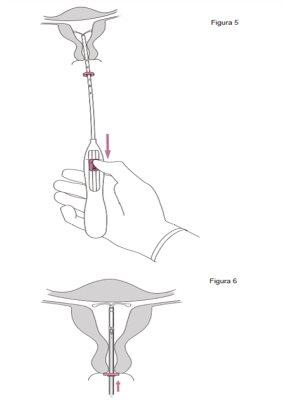
¡IMPORTANTE!No tirar de la corredera hacia abajo porque esto puede liberar Jaydess prematuramente. Una vez liberado, Jaydess no se puede volver a cargar.
|
|
¡IMPORTANTE!No forzar el insertor. Dilatar el canal cervical si es necesario. |
|
|
|
|
¡IMPORTANTE!Si se sospecha que el sistema no está en la posición correcta, comprobar su ubicación (p. ej. mediante ecografía). Extraer el sistema si no está bien colocado dentro de la cavidad uterina. No debe reinsertarse un sistema extraído.
Extracción/sustitución
Para obtener información sobre la extracción/sustitución, consultar la ficha técnica de Jaydess.
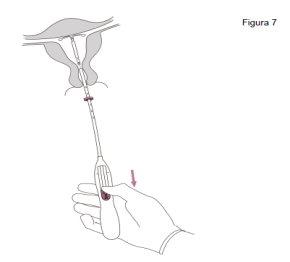
Jaydess se extrae tirando suavemente de los hilos con pinzas. Se puede insertar un nuevo Jaydess inmediatamente después de la extracción. Después de la extracción de Jaydess, debe examinarse el sistema para asegurar que está intacto y que se ha extraído por completo. |
|
Inclusión a nivel nacional del código QR que le dirige a la Ficha Técnica
La Ficha Técnica de Jaydess está disponible en la dirección de internet https://cima.aemps.es/info/77169
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a JAYDESS 13,5 MG SISTEMA DE LIBERACION INTRAUTERINOForma farmacéutica: DISPOSITIVO INTRAUTERINO, 19,5 mgPrincipio activo: plastic IUD with progestogenFabricante: Bayer Hispania S.L.Requiere recetaForma farmacéutica: DISPOSITIVO INTRAUTERINO, 52 mg/ tasa de liberación inicial de 0,02 mg cada 24 hPrincipio activo: plastic IUD with progestogenFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: DISPOSITIVO INTRAUTERINO, 0,02 mg/24 hPrincipio activo: plastic IUD with progestogenFabricante: Gedeon Richter Plc.Requiere receta
Médicos online para JAYDESS 13,5 MG SISTEMA DE LIBERACION INTRAUTERINO
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de JAYDESS 13,5 MG SISTEMA DE LIBERACION INTRAUTERINO, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes