
IXIARO SUSPENSION INYECTABLE

Cómo usar IXIARO SUSPENSION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
IXIARO suspensión inyectable
Vacuna contra la encefalitis japonesa (inactivada, adsorbida)
Lea todo el prospecto detenidamente antes de que usted o su hijo empiece a usar esta vacuna, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede que usted y su hijo tengan que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Esta vacuna se le ha recetado solamente a usted y/o a su hijo. No debe dársela a otras personas.
- Si usted y/o su hijo experimentan algún efecto adverso, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es IXIARO y para qué se utiliza
- Qué necesita saber antes de que usted y/o su hijo usen IXIARO
- Cómo se administra IXIARO
- Posibles efectos adversos
- Conservación de IXIARO
- Contenido del envase e información adicional
1. Qué es IXIARO y para qué se utiliza
IXIARO es una vacuna contra el virus de la encefalitis japonesa.
La vacuna consigue que el organismo cree su propia protección (anticuerpos) contra esta enfermedad.
IXIARO está indicado para prevenir la infección por el virus de la encefalitis japonesa (VEJ). Este virus se encuentra principalmente en Asia y se transmite al ser humano a través de mosquitos que han picado a un animal infectado (por ejemplo, un cerdo). Muchas personas infectadas presentan síntomas leves o no tienen síntomas. En las personas que contraen una forma grave de la enfermedad, la EJ suele empezar de forma parecida a la gripe, con fiebre, escalofríos, cansancio, dolor de cabeza, náuseas y vómitos. En las fases iniciales de la enfermedad aparecen también confusión y agitación.
IXIARO solo debe administrarse a adultos, adolescentes, niños o bebés mayores de 2 meses que vayan a viajar a países en los que la EJ sea endémica o que, por su trabajo, estén expuestos al riesgo de contraerla.
2. Qué necesita saber antes de que usted y/o su hijo empiecen a usar IXIARO
NO use IXIARO:
- Si usted y/o su hijo son alérgicos (hipersensibles) al principio activo o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si usted y/o su hijo han experimentado una reacción alérgica después de recibir una dosis anterior de IXIARO. Los síntomas de una reacción alérgica pueden consistir en una erupción cutánea con picor, dificultad para respirar e hinchazón de la cara y la lengua.
- i usted y/o su hijo tienen una enfermedad que cursa con fiebre alta. En este caso, su médico pospondrá la vacunación.
Advertencias y precauciones
IXIARO no debe inyectarse en un vaso sanguíneo.
La primovacunación debe completarse al menos una semana antes de la posible exposición al VEJ.
Informe a su médico:
- Si usted y/o su hijo han experimentado algún problema de salud después de la administración de alguna vacuna anterior;
- Si usted y/o su hijo tienen alguna otra alergia conocida;
- Si sufren un trastorno hemorrágico (enfermedad que le haga a usted y/o a su hijo sangrar más de lo normal) o presentan una disminución de las plaquetas sanguíneas, lo que aumenta el riesgo de hemorragia o de hematomas (trombocitopenia);
- Si su hijo tiene menos de 2 meses de edad, dado que IXIARO no se ha probado en bebés menores de 2 meses;
- Si su sistema inmunitario (de usted y/o de su hijo) no funciona correctamente (inmunodeficiencia) o si usted y/o su hijo están tomando medicamentos que afectan a su sistema inmunitario (como un medicamento llamado cortisona o medicamentos para el cáncer).
Su médico le explicará los posibles riesgos y beneficios de recibir IXIARO.
Tenga en cuenta lo siguiente:
- IXIARO no puede causar la enfermedad frente a la que protege.
- IXIARO no evitará infecciones causadas por otros virus distintos al de la encefalitis japonesa.
- Como cualquier otra vacuna, la vacunación con IXIARO puede no proporcionar protección en todos los casos.
- Debe tomar las precauciones necesarias para evitar que usted y/o su hijo reciban picaduras de mosquitos (uso de prendas adecuadas, repelentes, mosquiteras) incluso después de haberse vacunado con IXIARO.
Uso de IXIARO con otros medicamentos
Estudios realizados en seres humanos para evaluar la eficacia y la seguridad de los medicamentos (ensayos clínicos) han demostrado que IXIARO puede administrarse al mismo tiempo que la vacuna contra la hepatitis A y la vacuna contra la rabia.
Informe a su médico si usted y/o su hijo están utilizando, han utilizado recientemente o podrían tomar otros medicamentos, incluso los adquiridos sin receta, o si ha recibido recientemente alguna otra vacuna.
Embarazo, lactancia y fertilidad
No existen datos suficientes sobre la utilización de IXIARO en mujeres embarazadas o en periodo de lactancia.
Como medida de precaución, IXIARO no debería utilizarse durante el embarazo o la lactancia.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar esta vacuna.
Conducción y uso de máquinas
IXIARO no influye o su influencia es insignificante sobre la capacidad para conducir y utilizar máquinas.
IXIARO contiene potasio y sodio
Este medicamento contiene potasio, menos de 1 mmol (39 mg) por cada 0,5 ml de dosis única, esto es, esencialmente “libre de potasio”) y menos de 1 mmol de sodio (23 mg) por cada 0,5 ml de dosis única, esto es, esencialmente “libre de sodio”). Este medicamento puede contener restos de metabisulfito de sodio que están por debajo del límite de detección
3. Cómo usar IXIARO
La dosis recomendada para adultos, adolescentes y niños mayores de 3 años es de 2 inyecciones de 0,5 ml cada una:
- La primera inyección, el día 0
- La segunda inyección, 28 días después de la primera (día 28)
Los adultos de 18 a ≤ 65 años también pueden vacunarse de la siguiente forma:
- La primera inyección el día 0
- La segunda inyección siete días después de la primera inyección (día 7).
Bebés y niños con edades entre 2 meses y <3 años de edad
La dosis recomendada para los bebés y los niños con edades entre 2 meses y <3 años de edad es de 2 inyecciones de 0,25 ml cada una:
- La primera inyección, el día 0
- La segunda inyección, 28 días después de la primera (día 28)
Para obtener instrucciones acerca de la preparación de la dosis de 0,25 ml, consulte la parte final de este prospecto.
Asegúrese de que usted y/o su hijo finalizan el programa completo de vacunación de 2 inyecciones. La segunda inyección deberá administrarse al menos una semana antes de la posible exposición al virus de la encefalitis japonesa. De lo contrario, usted y/o su hijo podrían no quedar totalmente protegidos frente a la enfermedad.
En el caso de los adultos, adolescentes, niños y lactantes de 1 año o mayores, puede administrarse una dosis de recuerdo durante el segundo año (es decir, 12 a 24 meses ) después de la primera dosis de la inmunización primaria recomendada. En el caso de los adutos, se puede admnistrar una segunda dosis de recuerdo 10 años después de la primera dosis de recuerdo. En el caso de los personas de edad avanzada (> 65 años) la primera dosis de recuerdo puede administrarse antes. Su médico decidirá acerca del requisito y el momento de las dosis de recuerdo.
Administración
El médico o una enfermera le inyectarán IXIARO a usted y/o a su hijo en el músculo del brazo (deltoides). IXIARO no debe inyectarse en un vaso sanguíneo. En caso de que usted y/o a su hijo sufran un trastorno hemorrágico, es posible que su médico decida administrarle la vacuna por debajo de la piel (vía subcutánea).
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
Si olvidó recibir IXIARO
Si usted y/o a su hijo se saltan una de las inyecciones programadas, consulte a su médico y pida otra cita para la segunda inyección. Sin la segunda inyección, usted y/o a su hijo no estarán completamente protegidos contra la enfermedad. Hay información que indica que la segunda inyección puede administrarse hasta 11 meses después de la primera.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este producto puede producir efectos adversos, aunque no todas las personas los sufran.
Durante los ensayos clínicos se han observado la mayoría de los siguientes efectos adversos, que suelen producirse en los tres días siguientes a la vacunación, son por lo general leves y desaparecen al cabo de unos días.
Muy frecuentes (afectan a más de 1 usuario de cada 10):
Dolor de cabeza, dolor muscular, dolor en el lugar de la inyección, hipersensibilidad en el lugar de la inyección, cansancio
Frecuentes (afectan a entre 1 y 10 usuarios de cada 100):
Náuseas, enfermedad parecida a la gripe, fiebre, otras reacciones en el lugar de inyección (por ej., enrojecimiento, endurecimiento, hinchazón, picor)
Poco frecuentes (afectan a entre 1 y 10 usuarios de cada 1000):
Vómitos, erupción cutánea, cambios en los ganglios linfáticos, migraña (dolor de cabeza palpitante, a menudo acompañado de náuseas y vómitos y sensibilidad a la luz), mareo, vértigo (sensación de que todo da vueltas), diarrea, dolor abdominal, sudoración excesiva, picor, escalofríos, sensación de malestar general, rigidez musculoesquelética, dolor en las articulaciones, debilidad, resultados anormales de las pruebas del hígado (aumento de las enzimas hepáticas)
Raros (afectan a entre 1 y 10 usuarios de cada 10.000):
Palpitaciones, ritmo del corazón rápido, dificultad para respirar, sensación anormal en la piel (por ejemplo pinchazos), ronchas, enrojecimiento de la piel, dolor en las piernas o los brazos, deficiencia de plaquetas, inflamación de los nervios, hinchazón de las extremidades y los tobillos, alteración del gusto, hinchazón en los párpados, desmayo
Otros efectos adversos en niños con edades entre 2 meses y <3 años
Entre los niños con edades entre 2 meses y <3 años se han observado con mayor frecuencia los siguientes efectos adversos, en comparación con los niños de entre 3 y <12 años, los adolescentes y los adultos:
Muy frecuentes:fiebre (28,9 %), diarrea (11,8 %), enfermedad parecida a la gripe (11,2 %), irritabilidad (11,0 %)
Frecuentes:pérdida del apetito, vómitos, exantema
Poco frecuentes:tos
Comunicación de efectos adversos
Si usted y/o su hijo experimentas en cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de IXIARO
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
- Conservar en nevera (entre 2 ºC y 8 ºC).
- No congelar. Si la vacuna se ha congelado, no debe utilizarse.
- Conservar en el embalaje original para protegerlo de la luz.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los medicamentos que usted y/o su hijo ya no necesitan. De esta forma, ayudará a proteger el medio ambiente.
6. INFORMACIÓN ADICIONAL
Composición de IXIARO
Una dosis (0,5 ml) de IXIARO contiene:
6 AU3 de la cepa SA14-14-2 (inactivada) del virus de la encefalitis japonesa1,2
equivalente a una potencia ≤ 460 ng DE50
- producida en células Vero
- adsorbida en hidróxido de aluminio hidratado (aproximadamente 0,25 miligramos Al3+)
- Unidades de antígeno
El hidróxido de aluminio se añade a esta vacuna como adyuvante.
Los demás componentes son: cloruro sódico, dihidrógeno fosfato de potasio, hidrógeno fosfato disódico, agua para preparaciones inyectables
Aspecto del producto y contenido del envase
IXIARO es una suspensión inyectable (0,5 ml en una jeringa de vidrio con o sin aguja suministrada por separado, envase con 1 jeringa).
IXIARO es una suspensión estéril de color blanco o ligeramente lechoso que se vuelve homogénea cuando se agita.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Valneva Austria GmbH
Campus Vienna Biocenter 3
A-1030 Viena
Austria
E-mail: [email protected]
Responsable de la fabricación:
Valneva Scotland Ltd.
Oakbank Park Road,
Livingston EH53 0TG
Reino Unido
Valneva Austria GmbH
Campus Vienna Biocenter 3
A-1030 Viena
Austria
Para cualquier información sobre este producto , por favor, contacte con el titular de la autorización de comercialización en la siguiente dirección:
Fecha de la última revisión de este prospecto.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/. En la página web de la Agencia Europea de Medicamentos puede encontrarse este prospecto en todas las lenguas de la Unión Europea/Espacio Económico Europeo.
--------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
La jeringa precargada es de un solo uso y no debe ser utilizada en más de una persona. La jeringa precargada está lista para utilizar. Si no se suministra una aguja, utilice una aguja estéril.
No utilizar si la lámina del blíster no está intacta o si el envase está dañado.
Durante el período de conservación, puede que se forme un ligero depósito blanco con un sobrenadante incoloro y transparente.
Antes de la administración, agitar bien la jeringa para obtener una suspensión blanca, opaca y homogénea. No administrar si se observa la presencia de partículas o cambios de color tras el agitado o si la jeringa presenta daños físicos.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
Información acerca de la administración de una dosis de 0,5 ml de IXIARO a personas mayores de 3 años
En el caso de la administración de una dosis completa de 0,5 ml siga los siguientes pasos:
- Agitar la jeringa hasta obtener una suspensión homogénea.
- Retirar la tapa de la punta de la jeringa girándola suavemente. No intente romper ni tirar de la punta porque eso podría dañar la jeringa.
- Acoplar una aguja a la jeringa precargada.
Información acerca de la preparación de una dosis de 0,25 ml de IXIARO para utilizarla en niños menores de 3 años
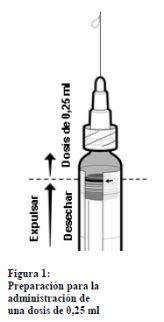
En el caso de la administración de una dosis de 0,25 ml a niños con edades entre 2 meses y <3 años siga los siguientes pasos:
- Agitar la jeringa hasta obtener una suspensión homogénea.
- Retirar la tapa de la punta de la jeringa girándola suavemente. No intente romper ni tirar de la punta porque eso podría dañar la jeringa.
- Acoplar una aguja a la jeringa precargada.
- Mantener la jeringa en posición vertical.
- Empujar el tapón del émbolo hasta el borde de la línea roja del tubo de la jeringa, indicada con una flecha roja (ver la figura 1)* para desechar el volumen sobrante
- Acoplar una nueva aguja estéril antes de inyectar el volumen restante.
*Si empuja el tapón del émbolo más allá de la línea roja, no se garantiza la dosis de 0,25 ml y habrá que utilizar una jeringa nueva.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a IXIARO SUSPENSION INYECTABLEForma farmacéutica: INYECTABLE, 60 microgramos/dosis + 60 microgramos/dosisPrincipio activo: respiratory syncytial virus vaccinesFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: INYECTABLE, 3,75 microgramosPrincipio activo: influenza, inactivated, split virus or surface antigenFabricante: Glaxosmithkline BiologicalsRequiere recetaForma farmacéutica: INYECTABLE, DesconocidaPrincipio activo: combinationsFabricante: Glaxosmithkline BiologicalsRequiere receta
Médicos online para IXIARO SUSPENSION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de IXIARO SUSPENSION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















