
IPRATROPIO BROMURO/SALBUTAMOL CIPLA 0,5 MG/2,5 MG SOLUCION PARA INHALACION POR NEBULIZADOR


Cómo usar IPRATROPIO BROMURO/SALBUTAMOL CIPLA 0,5 MG/2,5 MG SOLUCION PARA INHALACION POR NEBULIZADOR
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Ipratropio bromuro/salbutamol Cipla y para qué se utiliza
- Que necesita saber antes de empezar a usar Ipratropio bromuro/salbutamol Cipla
- Cómo usar Ipratropio bromuro/Salbutamol Cipla
- Posibles efectos adversos
- Conservación de Ipratropio bromuro /Salbutamol Cipla
- Contenido del envase e información adicional
Introducción
Prospecto: Información para el usuario
Ipratropio bromuro/Salbutamol Cipla 0,5mg/2,5mg solución para inhalación por nebulizador
Bromuro de ipratropio / salbutamol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ipratropio bromuro/Salbutamol Cipla y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ipratropio bromuro/Salbutamol Cipla
- Cómo usar Ipratropio bromuro/Salbutamol Cipla
- Posibles efectos adversos
- Conservación de Ipratropio bromuro/Salbutamol Cipla
- Contenido del envase e información adicional
1. Qué es Ipratropio bromuro/salbutamol Cipla y para qué se utiliza
Este medicamento se llama Ipratroprio bromuro/Salbutamol Cipla. Los principios activos son bromuro de ipratropio y salbutamol. Bromuro de ipratropio y salbutamol pertenecen a un grupo de medicamentos llamados broncodilatadores, que ayudan a mejorar la respiración abriendo las vías respiratorias. Lo hacen evitando la contracción de los músculos lisos que rodean las vías respiratorias, de modo que éstas permanecen abiertas. Bromuro de ipratropio actúa bloqueando las señales nerviosas dirigidas a los músculos que rodean las vías respiratorias, y salbutamol actúa estimulando los receptores β2 en los músculos.
Ipratropio bromuro/Salbutamol Cipla se utiliza para tratar problemas respiratorios en pacientes mayores de 12 años con dificultades respiratorias de larga duración (enfermedad pulmonar obstructiva crónica como bronquitis crónica, enfisema). Ipratropio bromuro/Salbutamol Cipla aliviará las sibilancias , la dificultad para respirar y la opresión en el pecho.
Este medicamento se utiliza con un aparato llamado «nebulizador», que transforma el medicamento en forma de vapor para que lo pueda respirar.
2. Que necesita saber antes de empezar a usar Ipratropio bromuro/salbutamol Cipla
No use Ipratropio bromuro/Salbutamol Cipla:
- si es alérgico al salbutamol, bromuro de ipratropio, atropina (incluyendo los medicamentos similares a la atropina) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6),
- si padece cardiomegalia (corazón agrandado) o una enfermedad llamada miocardiopatía hipertrófica obstructiva o MHO,
- si padece ritmo cardíaco rápido (taquicardias),
Advertencias y precauciones
Consulte a su médico antes de empezar a usar ipratropio/salbutamol:
- si padece o cree que podría padecer una afección ocular denominada glaucoma (aumento de la presión en los ojos) o cualquier otra afección ocular. Su médico quizá le aconseje que se proteja los ojos cuando utilice este medicamento.
- si es un hombre y tiene una hipertrofia prostática o problemas para orinar.
- si ha tenido un ataque al corazón (infarto de miocardio) recientemente.
- si sufre problemas arteriales o dolor en las piernas cuando camina.
- si tiene antecedentes de enfermedad cardíaca, ritmo cardíaco irregular o angina de pecho (por favor informe a su médico antes de comenzar a utilizar este medicamento).
- si padece diabetes.
- si tiene una glándula tiroides hiperactiva.
- si padece fibrosis quística.
- si le han comunicado que tiene un tumor en la glándula suprarrenal.
- si tiene un trastorno denominado feocromocitoma, que es un tumor poco frecuente y no maligno. El uso del inhalador puede empeorar los símptomas del mismo.
- si accidentalmente le entra líquido o neblina en los ojos, puede sentir dolor, picor o enrojecimiento, pupilas dilatadas, visión borrosa, ver colores o luces. Si esto sucede, consulte a su médico. Si presenta problemas en los ojos en cualquier otro momento, consulte a su médico.
Se han notificado casos de caries dentales con el uso de salbutamol. Se recomienda, especialmente en los niños, prestar atención a la higiene bucal adecuada y realizar revisiones dentales periódicas.
Se ha observado acidosis láctica asociada a altas dosis terapéuticas de salbutamol, principalmente en pacientes tratados con un broncoespasmo agudo (ver las secciones 3 y 4). El aumento en los niveles de lactato puede dar lugar a la falta de respiración e hiperventilación. Hable inmediatamente con su médico si usted siente que el medicamento no está funcionando como habitualmente y necesita usar el nebulizador más veces de las que su médico le ha recomendado.
Consulte a su médico en caso de empeoramiento repentino de sus trastornos respiratorios o si la dosis prescrita no da un resultado normal. No aumente la dosis sin consultar al médico.
Si utiliza dosis elevadas de ipratropio/salbutamol durante mucho tiempo, se debe vigilar la cantidad de potasio en sangre, especialmente si está tomando otros medicamentos al mismo tiempo, por ejemplo: esteroides (corticoesteroides), medicamentos que aumentan la producción de orina (diuréticos) u otros medicamentos que abran las vías respiratorias, como teofilina (xantinas).
Niños y adolescentes
Este medicamento no debe utilizarse en niños menores de 12 años.
Otros medicamentos e Ipratropio bromuro/Salbutamol Cipla
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Determinados medicamentos pueden interactuar con bromuro de ipratropio/salbutamol y empeorar los efectos adversos o reducir el efecto de ipratropio/salbutamol. Informe siempre a su médico si está tomando alguno de los siguientes medicamentos:
- Otros medicamentos que ayudan a respirar, como salbutamol y preventivos como dipropionato de beclometasona. Estos pueden aumentar el efecto de bromuro de ipratropio/salbutamol e incrementar la intensidad de los efectos adversos.
- Betabloqueantes, es decir, medicamentos utilizados a menudo para tratar afecciones cardíacas, como el dolor en el pecho que se produce durante el esfuerzo (llamado angina de pecho), los latidos cardíacos irregulares (o arritmias) y la presión arterial alta (llamada hipertensión). Algunos de ellos, como propranolol, pueden reducir la cantidad de potasio en sangre cuando se administran al mismo tiempo que bromuro de ipratropio/salbutamol (los betabloqueantes pueden reducir el efecto del salbutamol).
- Determinados medicamentos para tratar la depresión («antidepresivos»). En esta clase de medicamentos se incluyen los inhibidores de la monoaminooxidasa (p. ej., fenelzina) o los antidepresivos tricíclicos (p. ej., amitriptilina).
- Digoxina (para problemas del corazón) puede causar problemas del ritmo cardíaco cuando se administra junto con bromuro de ipratropio/salbutamol.
- Medicamentos llamados «anticolinérgicos». Se emplean para tratar el dolor cólico, la enfermedad de Parkinson, los problemas para orinar o la incontinencia urinaria o fecal.
- Una reducción de la cantidad de potasio en la sangre (hipopotasemia) debida al salbutamol de ipratropio/salbutamol es más probable si está utilizando bromuro de ipratropio/salbutamol junto con otros tratamientos para el asma, junto con corticoesteroides inhalados o en comprimidos (como la prednisona), o junto con diuréticos (para incrementar la eliminación de orina). Un nivel bajo de potasio en sangre puede causar debilidad muscular, contracciones musculares o anomalías del ritmo cardíaco. Es posible que su médico le haga un análisis de sangre para medir la cantidad de potasio de vez en cuando.
- Los anestésicos pueden aumentar la propensión a padecer los efectos adversos de salbutamol sobre el corazón. Su médico le supervisará estrechamente o quizá decida interrumpir el tratamiento conipratropium/salbutamol si le van a operar.
Si le van a administrar anestesia general en el hospital, informe al anestesista de los medicamentos que está tomando.
Uso de Ipratropio bromuro/Salbutamol Cipla con alimentos y bebidas
Los alimentos y las bebidas no ejercen ninguna influencia sobre este medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No use ipratropio/salbutamol si está embarazada, a menos que su médico decida que el beneficio para usted es superior al riesgo para su hijo.
Este medicamento se puede utilizar durante la lactancia. Consulte a su médico, farmacéutico o enfermero antes de utilizar este medicamento durante la lactancia.
Conducción y uso de máquinas
Si padece efectos adversos como mareos, dificultad para enfocar la vista y visión borrosa durante el tratamiento con ipratropio/salbutamol, debe evitar tareas potencialmente peligrosas como conducir o utilizar máquinas.
Ipratropio bromuro/Salbutamol Cipla contiene sodio
Este medicamento contiene menos de 1 mmol (23 mg) de sodio por dosis, por lo que se considera esencialmente «exento de sodio».
3. Cómo usar Ipratropio bromuro/Salbutamol Cipla
Este medicamento es para uso por inhalación. La solución para inhalación por nebulizador es para inhalación oral tras la nebulización.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte a su médico o enfermero.
Este medicamento se debe utilizar a demanda y no de forma regular.
Si su asma está activa (por ejemplo, tiene síntomas o crisis frecuentes, como dificultad para respirar que le dificulta hablar, comer o dormir, tos, sibilancias, opresión en el pecho o capacidad física limitada), debe informar inmediatamente a su médico, que le puede empezar a administrar un medicamento o aumentar la dosis de tratamiento, como un corticosteroide inhalado, para controlar su asma.
Informe a su médico lo antes posible si su medicamento parece no estar funcionando tan bien como de costumbre (por ejemplo, si necesita dosis más altas para aliviar sus problemas respiratorios o si su inhalador no le proporciona alivio durante al menos 3 horas), ya que su asma puede estar empeorando y usted puede necesitar un medicamento diferente.
Si utiliza este medicamento más de dos veces por semana para tratar sus síntomas asmáticos, sin incluir el uso preventivo antes del ejercicio, esto indica un asma mal controlada y puede aumentar el riesgo de ataques de asma graves (empeoramiento del asma) que pueden tener complicaciones serias y pueden poner en riesgo su vida o incluso ser mortales. Se debe poner en contacto con su médico lo antes posible para revisar su tratamiento del asma.
Si utiliza a diario un medicamento contra la inflamación de sus pulmones, p.ej., un “corticosteroide inhalado”, es importante que siga utilizándolo con regularidad, aunque se sienta mejor.
La dosis recomendada para adultosy niños mayores de 12añoses de 1 ampolla, de tres a cuatro veces al día.
Los pacientes de edad avanzada deben tomar la dosis habitual de adultos.
Uso en niños
Nose recomienda el uso de este ipratropio/salbutamol en niños menores de 12 años.
No tragar o administrar este medicamento medicante inyección.
En la etiqueta encontrará la cantidad que debe tomar y con qué frecuencia.
No utilice una cantidad de medicamento superior a la indicada por su médico. Informe a su médico si sus problemas respiratorios empeoran, si el medicamento no le alivia los problemas para respirar tanto como antes o si está utilizando el inhalador azul «de rescate» de acción corta (de alivio rápido) con más frecuencia de lo habitual.
Este medicamento se debe utilizar con un nebulizador apto, p. ej., PARI LC PLUS®, nebulizador neumático. Lea detenidamente las instrucciones de uso del nebulizador en el prospecto de PARI LC PLUS® antes de comenzar la inhalación.
Instrucciones de uso
- Prepare el nebulizador siguiendo las instrucciones del fabricante y el consejo de su médico.
- Abra la bolsa y saque la tira de ampollas de dosis unitaria.
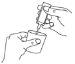
- Separe con cuidado una ampolla de la tira etiquetada girando y tirando. No utilice nunca una ampolla que ya haya sido abierta o si la solución para inhalación por nebulizador está descolorida (diagrama A).
- No la use si ésta ya ha sido abierta o si el líquido del interior está descolorido.
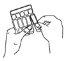
- Mantenga la ampolla en posición vertical y ábrala girando la parte superior (diagrama B).
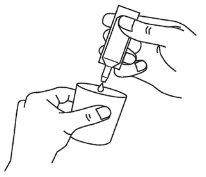
- Transfiera todo el contenido a la cámara del nebulizador apretando la ampolla (diagrama C).
- Ensamble el nebulizador y úselo siguiendo las instrucciones del fabricante y las indicaciones de su médico.
- Si su médico le ha indicado que su medicamento necesita ser diluido, le darán una solución estéril de cloruro sódico al 0,9%. Su médico le explicará que debe hacer.
- Después de utilizar el nebulizador, tire toda la solución que haya quedado en la cámara. Si queda solución para inhalación por nebulizador en la ampolla, también debe desecharse.
- Limpie bien el nebulizador siguiendo las instrucciones del fabricante.
A | B | C |

No diluya la solución para inhalación por nebulizador ni la mezcle con otros medicamentos, a menos que se lo indique su médico.
Las ampollas de dosis única de este medicamento no contienen conservantes y, por ello, es importante que el contenido se utilice inmediatamente después de la apertura. Debe utilizar una ampolla nueva para cada administración de ipratropio/salbutamol con el nebulizador.
Deben desecharse las ampollas parcialmente utilizadas, abiertas o dañadas. No debe utilizar nuncauna ampolla que ya haya sido abierta.
Es importante que siga estas instrucciones para evitar la contaminación de la solución para inhalación por nebulizador contenida en las ampollas.
Noingiera la solución para inhalación por nebulizador ni la utilice en inyecciones.
Nopermita que la solución para inhalación por nebulizador o el vapor entre en contacto con los ojos. Si alguno de los líquidos o el vapor le entra en los ojos accidentalmente, es posible que le duelan, le escuezan o se le pongan rojos, que se le dilaten las pupilas, tenga visión borrosa o vea colores o luces. Si le sucediera, consulte a su médico. Si tiene problemas en los ojos en cualquier otro momento, consulte a su médico.
Si usa más Ipratropio bromuro/Salbutamol Cipla del que debe
Si ha tomado una dosis ligeramente superior a la habitual, quizá note un latido cardíaco más rápido (palpitaciones) o temblores. Otros síntomas pueden ser dolor en el pecho, cambios en la presión arterial, sofoco, inquietud o mareos. Estos efectos suelen desaparecer en unas pocas horas. Es posible que descienda la cantidad de potasio en su sangre; su médico quizá desee vigilar su nivel de potasio haciéndole un análisis de sangre de vez en cuando. Informe a su médico si le preocupa alguno de estos síntomas o si estos persisten.
Si utiliza más cantidad de este medicamento de la que debe, informe a su médico de inmediato o acuda al hospital más cercano. Si decide acudir a un médico o al hospital, debe llevar todos sus medicamentos, incluidos los adquiridos sin receta médica; llévelos en su embalaje original si es posible. Lleve también este prospecto para mostrárselo al médico.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Ipratropio bromuro/Salbutamol Cipla
Si olvida tomar una dosis a la hora adecuada, tómela en cuanto se acuerde. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Ipratropio bromuro/Salbutamol Cipla
Su médico le indicará la duración del tratamiento conIpratropio/Salbutamol Cipla . No debe interrumpir el tratamiento con Ipratropio /Salbutamol Cipla sin consultar antes con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Algunos efectos adversos pueden ser graves y requerir intervención médica.
Efectos adversos graves
- Si su problema respiratorio o sus sibilancias empeoran inmediatamente después de inhalar este medicamento, o le cuesta respirar y se queda sin aliento, no tome más ipratropio/salbutamol; utilice en seguida su inhalador de alivio inmediato de acción corta. Debe dejar de utilizar ipratropio/salbutamol y ponerse en contacto con su médico cuanto antes. Es posible que su médico le prescriba otro tratamiento alternativo para su afección.
- Si cree que es alérgico a ipratropio/salbutamol o que está teniendo una reacción alérgica a la solución para inhalación por nebulizador (que incluyen hinchazón de la lengua, los labios y la cara), deje de usar este medicamento y contacte con su médico inmediatamente.
Otros efectos adversos pueden ocurrir con las siguientes frecuencias:
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Sequedad de boca
- Náuseas (ganas de vomitar)
- Irritación de la boca y la garganta
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- Dolor de cabeza
- Mareos
- Nerviosismo
- Temblores
- Sensación de mareo o de que todo da vueltas (vértigo)
- Palpitaciones (latido cardíaco fuerte)
- Latido cardíaco rápido
- Tos
- Irritación de garganta
- Problemas del habla
- Dificultad para orinar
- Reacciones cutáneas
Raros(pueden afectar hasta 1 de cada 1.000 personas)
- Reacción alérgica grave que causa dificultad para respirar o mareos
- Reacción alérgica con ronchas y picor
- Hinchazón de la cara, los labios y la lengua
- Disminución de la cantidad de potasio
- Trastornos mentales
- Sudoración
- Dolor en los ojos u otros problemas oculares, como visión borrosa, midriasis (dilatación excesiva de la pupila) y glaucoma (aumento de la presión en los ojos)
- Latidos irregulares del corazón
- Descenso de la presión arterial
- Insuficiencia cardíaca
- Problemas respiratorios y dificultad para respirar
- Hinchazón de la garganta
- Diarrea, estreñimiento, vómitos u otros problemas del aparato digestivo
- Alteración del gusto
- Caries dental
- Dolores musculares
- Debilidad y calambres
- Sequedad de garganta
- Edema bucal
- Estomatitis
- Sentirse débil
- Cambios de humor
- Dificultad para orinar
Muy raros(pueden afectar hasta 1 de cada 10.000 personas)
- Aumento de la presión arterial
Frecuencia no conocida(la frecuencia no puede ser estimada con los datos disponibles)
Una condición conocida como acidosis láctica que puede causar dolor de estómago, hiperventilación, dificultad respiratoria, a pesar de que pueda haber una mejoría en sus sibilancias, pies y manos fríos, latido del corazón irregular o sed.
Aunque se desconoce con qué frecuencia sucede exactamente, algunas personas podrían padecer dolor en el pecho (debido a problemas como la angina de pecho). Informe a su médico lo antes posible si padece estos síntomas mientras recibe tratamiento con ipratropio/salbutamol, pero no deje de usar este medicamento a menos que se lo indique su médico.
También puede presentar una cantidad inusualmente escasa de potasio en sangre («hipopotasemia»). Si presenta hipopotasemia, su médico continuará revisando sus niveles de potasio.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte con su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de uso humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ipratropio bromuro /Salbutamol Cipla
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja, el sobre y la etiqueta de la ampolla después de CAD. La fecha de caducidad es el último día del mes que se indica.
No refrigerar o congelar. No conservar a temperatura superior a 25°C.
De un solo uso. Utilizar inmediatamente después de abrir la ampolla por primera vez. Desechar inmediatamente después del primer uso.
La eliminación de las ampollas parcialmente utilizadas, abiertas o dañadas se realizará de acuerdo con la normativa local.
Conservar las ampollas en el envoltorio de aluminio o en el embalaje exterior para protegerlas de la luz.
No utilice este medicamento si observa que la solución para inhalación por nebulizador está turbia.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ipratropio bromuro/Salbutamol Cipla
- Los principios activos son bromuro de ipratropio y salbutamol. Cada ampolla de dosis única (dosis de 2,5 mL) contiene 0,5 mg de bromuro de ipratropio (equivalente a 525 microgramos de bromuro de ipratropio monohidrato) y 2,5 mg de salbutamol (como sulfato).
- Los demás excipientes son cloruro de sodio, agua para inyectables y ácido sulfúrico (para el ajuste del pH).
Aspecto del producto y contenido del envase
El envase de dosis única es una ampolla de polietileno que contiene 2,5 mL de solución para inhalación por nebulizador transparente e incolora.
Cinco ampollas de plástico en un envoltorio de aluminio con triple laminado (película de poliéster/lámina de aluminio/película de polietileno) y envasadas en cajas de cartón que contienen 10, 20, 40, 60, 80 o 100 ampollas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Cipla Europe NV
De Keyserlei 58-60, Box-19,
2018 Amberes
Bélgica
Responsable de la fabricación
Cipla Europe NV, De Keyserlei 58-60, Box-19, 2018, Antwerp, Bélgica
ó
ALTERNO LABS d.o.o., Brnciceva ulica 29, Ljubljana-Crnuce, 1231, Eslovenia
Representante local
Cipla Europe NV sucursal en España,
C/ Guzmán el Bueno, 133 Edif Britannia-28003- Madrid
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Países Bajos: Zerseos 0,5 mg/2,5 mg per 2,5 ml, verneveloplossing
Alemania: Ipratropium/Salbutamol Cipla 0,5 mg / 2,5 mg Lösung für eien Vernebler
España: Ipratropio bromuro/Salbutamol Cipla 0,5 mg/2,5 mg solución para inhalación por nebulizador
Irlanda: Zerseos 0,5 mg/2,5 mg per 2,5 ml nebuliser solution
Polonia: Ipratropium/Salbutamol Cipla, (0,5 mg + 2,5 mg)/2,5 ml, roztwór do nebulizacji
Fecha de la última revisión de este prospecto:enero 2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Precio medio en farmacia13.88 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a IPRATROPIO BROMURO/SALBUTAMOL CIPLA 0,5 MG/2,5 MG SOLUCION PARA INHALACION POR NEBULIZADORForma farmacéutica: INHALACIÓN PULMONAR, 0,5 mg/2,5 mgPrincipio activo: salbutamol and ipratropium bromideFabricante: Genetic S.P.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 0,5 mg/2,5 mgPrincipio activo: salbutamol and ipratropium bromideFabricante: Neutec Inhaler Ireland LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 0,5 mg/2,5 mgPrincipio activo: salbutamol and ipratropium bromideFabricante: Laboratorio Aldo Union S.L.Requiere receta
Médicos online para IPRATROPIO BROMURO/SALBUTAMOL CIPLA 0,5 MG/2,5 MG SOLUCION PARA INHALACION POR NEBULIZADOR
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de IPRATROPIO BROMURO/SALBUTAMOL CIPLA 0,5 MG/2,5 MG SOLUCION PARA INHALACION POR NEBULIZADOR, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















